The Use of Diphenyl Oxide Disulfonate Surfactants
in Emulsion Polymerization of Hydrophobic Monomers

Figure 1

Table 1

Table 2

Table 3
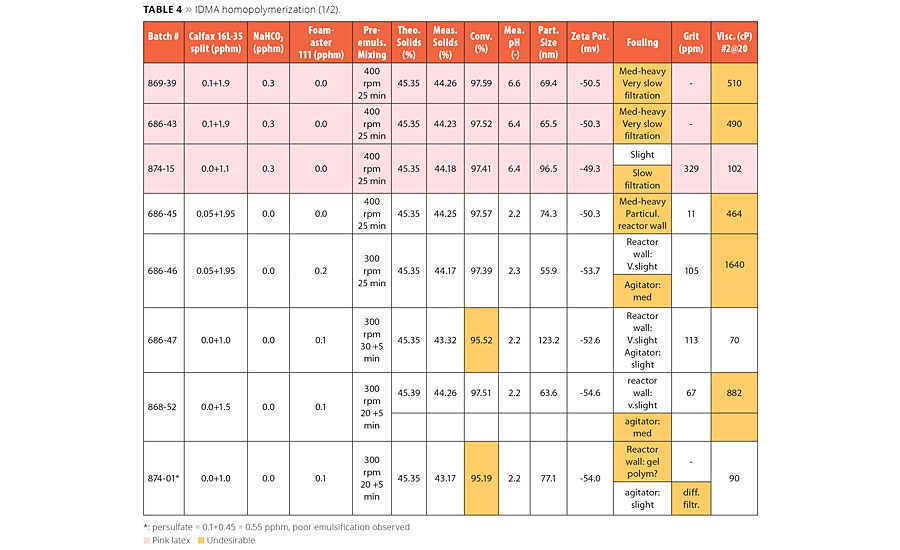
Table 4

Table 5
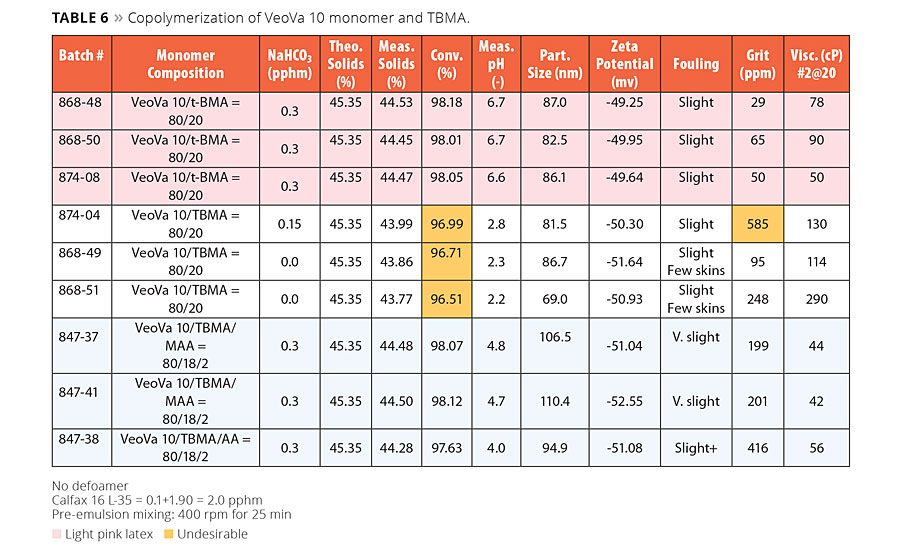
Table 6
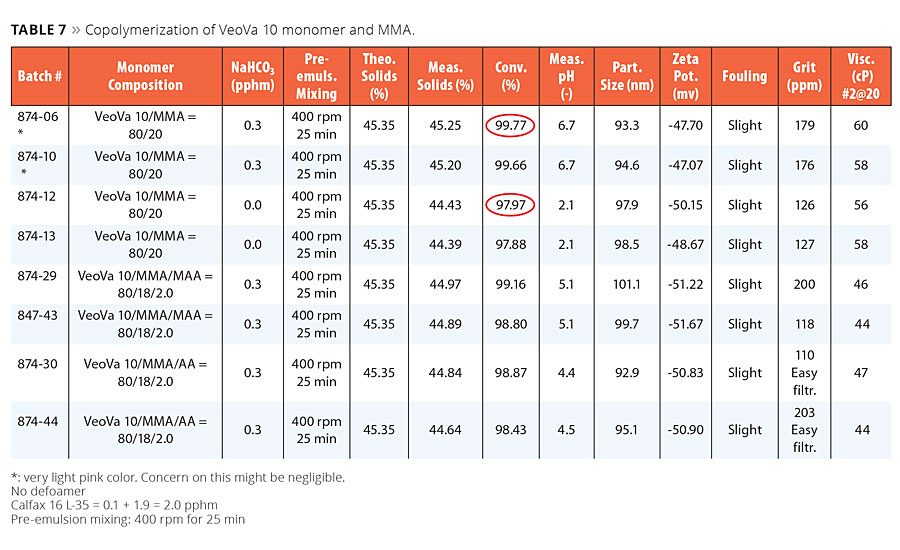
Table 7
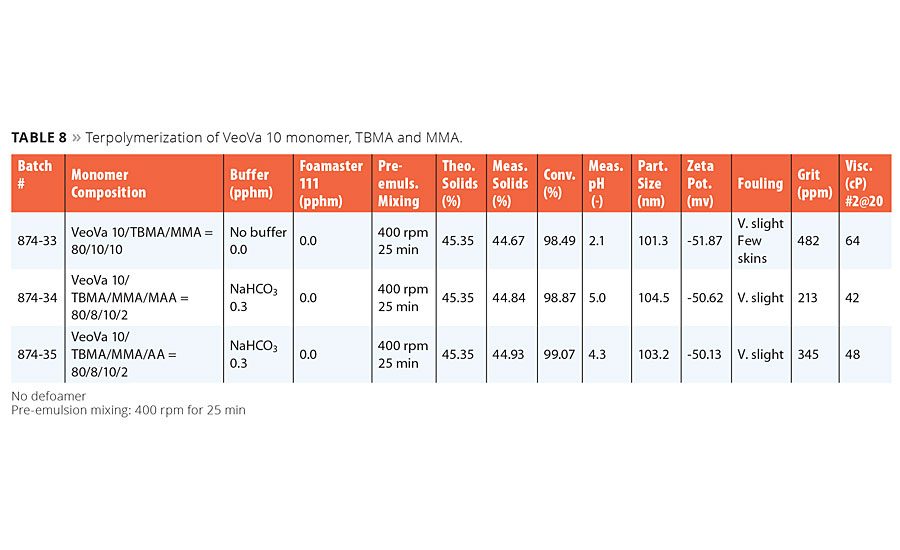
Table 8

Table 9

Table 10
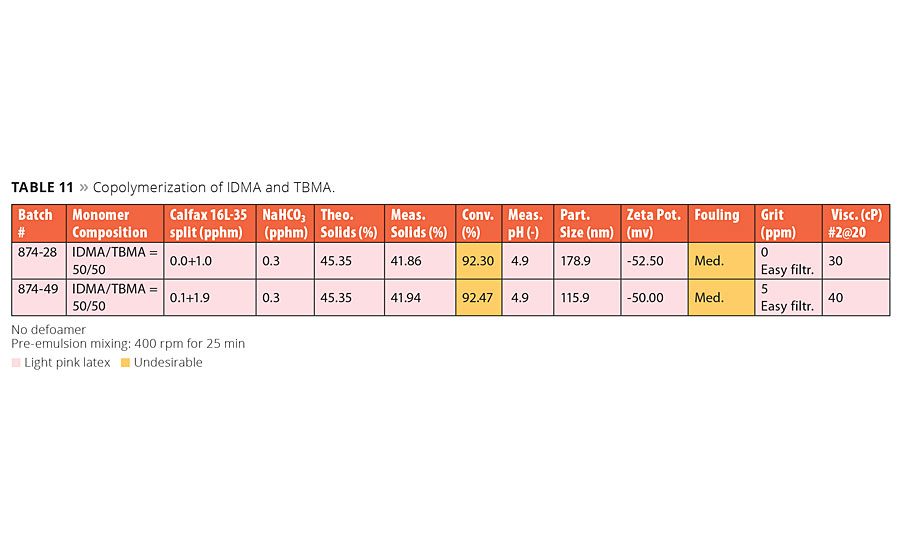
Table 11













One of the main requirements for protective coatings is the ability to confer water resistance to painted substrates. Since the binder is a major part of most coatings, ongoing research in emulsion polymer design is aimed at providing more effective barrier properties by increasing the hydrophobic nature of the polymers produced. That, in turn, requires a means for effectively and efficiently polymerizing hydrophobic monomers.
Attempts to make polymers of very hydrophobic monomers, such as those of long-chain acrylics or of vinyl branched esters that have carbon chain longer than C8, using common sulfonate surfactants have failed because of massive coagulation and/or very low conversion.1,2
Because of the inability to polymerize very hydrophobic monomers by emulsion polymerization, the coatings industry is forced to employ costly techniques such as high temperature and pressure; organic solvents or other monomers to act as solvents for the hydrophobic monomer; mini-emulsion polymerization; macromolecular organic compounds having a hydrophobic cavity, such as cyclodextrins; and using high levels of surfactants.2-5
A recent patent application1 claimed an emulsion polymerization process of vinyl branched esters with the use of at least one surfactant having a critical micelle concentration of less than 0.05%, which was specified as sodium bis-tridecyl sulfosuccinate. The drawback of this surfactant is that it contains ethanol, a flammable solvent; and its use as a single surfactant in emulsion polymerization might not be effective.
This article describes a simple emulsion polymerization process using a single diphenyl oxide disulfonate (DPOS) surfactant for polymerizing hydrophobic monomers, including VeoVa™ 10 monomer (Tg = -3 °C) and isodecyl methacrylate (IDMA) (Tg = -41 °C), using ammonium persulfate as the initiator. VeoVa 10 monomer is the vinyl ester of neodecanoic acid (10 carbon atoms), and isodecyl methacrylate is a C10 methacrylate. It also demonstrates promising copolymerization and terpolymerization of these hydrophobic monomers with harder monomers, such as methyl methacrylate (Tg = 105 °C), styrene (Tg = 100 °C) and tert-butyl methacrylate (TBMA, Tg = 107 °C), using the same surfactant and initiator.
Theoretical Consideration of Emulsion Polymerization of Hydrophobic Monomers
Emulsion polymerization is a useful technique in preparing aqueous dispersions or latexes, which are important components in coating systems.
For relatively hydrophobic monomers such as styrene or n-butyl methacrylate, it is generally accepted that the polymerization reaction takes place in monomer-swollen micelles. Only a small fraction of the monomer is present in the micelles or dissolved in the aqueous phase. Most of the monomer molecules reside in the monomer droplets. The polymerization is initiated by the addition of an initiator. Initiator free radicals first react with monomer molecules dissolved in the aqueous phase. This would result in the increased hydrophobicity of oligomeric radicals. When a critical chain length (“zmer”) is achieved, these oligomeric radicals become hydrophobic and show a strong tendency to enter the monomer-swollen micelles and then continue to propagate by reacting with those monomer molecules therein. As a consequence, monomer-swollen micelles are successfully transformed into particles. These particles continue to grow by acquiring the reactant species from monomer droplets and un-nucleated micelles. In order to maintain adequate colloidal stability of the growing particles, micelles that do not contribute to particle nucleation disband to supply the increasing demand for surfactant of the particles. In addition, the surfactant molecules adsorbed on monomer droplets may also desorb out of the droplet surface, diffuse across the aqueous phase and then adsorb on the expanding particle surface for particle stabilization.
Based on this understanding, we consider that, in order for the emulsion polymerization of hydrophobic monomers to proceed, both of the following two basic requirements must be satisfied:
1. The oligomeric (“zmer”) radicals must be generated in the water phase and enter the micelles to nucleate the particles, if a water soluble initiator such as persulfate is used. For this to happen, a certain amount of monomers must exist in the water phase.
2. The monomers have to effectively transport through the water phase to the particles to grow the particles.
Using DPOS Surfactants in Emulsion Polymerization of Hydrophobic Monomers
We made the following hypotheses:
1. The monomer concentration in water around the vicinity of 0.001% would be sufficient for the formation of oligomeric radicals. The “zmer” radical formation will be inhibited at monomer concentration far below 0.001%.
2. DPOS surfactant molecules have the base structure of alkylated DPO and contain in large part two sulfonate groups; they have excellent association with hydrophobic monomers and would be a good hydrophobic monomer transporter through the water phase to latex particles.
The first hypothesis was simply based on the water solubility data of VeoVa 10 monomer < 0.001 g/100 g at 20 °C (Table 1). We assumed the water solubility of IDMA was the same as that of VeoVa 10 monomer.
The production of DPOS surfactants in general can be briefly described by a simplified sketch (Figure 1).
The alkylated diphenyl oxide (alkylated DPO) intermediate contains two major isomers, mono alkylated DPO and dialkylated DPO. We consider that the alkyl chain length and the monoalkylate/dialkylate ratio might have an effect on the association with hydrophobic monomers, depending on the hydrophobicity of the monomer. The disulfonate content can also be adjusted for best emulsifier performance, depending on the nature of the alkylated DPO. Due to such importance mentioned, both the monoalkylate/dialkylate ratio and disulfonate content are controlled in a narrow range.
Study Strategy
Our exploring phase of studying the emulsion polymerization of hydrophobic monomers based on DPOS surfactants was designed to test the behavior and the effectiveness of major components used.
Surfactants
Three DPOS surfactants differentiated by the alkyl group were evaluated in this study: a) C12 branched, Calfax®DB-45; b) C15 branched, experimental Calfax product; c) C16 linear, Calfax 16L-35.
Initiator: Ammonium Persulfate
Ammonium persulfate or other persulfate salts are the most commonly used initiators in the emulsion polymerization industry today. The intent of this work was to test the hypothesis No.1 mentioned earlier, or the effectiveness of ammonium persulfate in the emulsion polymerization of VeoVa 10 monomer and isodecyl methacrylate. If the “zmer” radical formation is inhibited due to too low water solubility of the monomer, a search for alternative initiation system might become necessary.
Monomers
a. VeoVa 10 monomer (Tg = -3 °C) and isodecyl methacrylate (C10 methacrylate, Tg = -41 °C) were the two major hydrophobic monomers used in this study.
b. Methyl methacrylate (Tg = 105 °C), styrene (Tg = 100 °C) and t-butyl methacrylate (Tg = 107 °C) were used as hard monomers for the copolymerization and terpolymerization with VeoVa 10 monomer or isodecyl methacrylate.
It should be noted that VeoVa 10 vinyl ester and styrene do not react with each other. Incorporation of 2.0 pphm acid monomer such as acrylic acid (Tg = 106 °C) or methacrylic acid (Tg = 220 °C) into copolymers or terpolymers was also investigated.
c. Lauryl acrylate (Tg = -3 °C), lauryl methacrylate (Tg = -65 °C), stearyl methacrylate (Tg = 38 °C) are to be used in the next step for examining the behavior of initiator and DPOS surfactant – C16 surfactant in extreme hydrophobicity conditions.
d. All the monomers were used as supplied, without -purification.
Emulsion Polymerization Protocol
All latexes were made in a 1L jacketed glass reactor with four built-in baffles. The agitation of the reaction mixture was conducted using a stirrer system including a PTFE covered stainless steel shaft and two 64-mm turbine PTFE blades. The temperature was regulated by an external circulating water bath with heating and cooling capability. The reactor was fitted with a thermometer and feed lines. Both monomer pre-emulsion and initiator solution were fed via FMI pumps and above the surface of the reaction mixture.
For easy comparison, all emulsion polymerization (EP) experiments in this study were based on the same EP procedure and process conditions. The emulsion polymerization experimentation can be summarized as follows:
1. EP process conditions:
- In-situ seed with 2% pre-emulsion.
- Reaction temperature: 80 ± 1 °C.
- Pre-emulsion feed time: 3.0 hrs.
- Initiator solution feed time: 3.5 hrs.
- Hold time after initiator feed: 0.5 hr.
2. Pre-emulsion preparation:
- Mixing time: 25 min.
- Mixing speed: 400 rpm was found most appropriate. However, the mixing speed was recorded and will be shown in the results.
- In case buffer is used, buffer solution was added to surfactant solution at mild agitation (at 150 -200 rpm) before the addition of monomer, and the agitation was then made at higher speed.
- When defoamer was used, defoamer was added to the pre-emulsion while mixing and 5 min before completion.
3. Monomer activity:
All monomers were assumed to have activity of 100% in all experiments, except for the copolymerization or terpolymerization of IDMA with other monomers, the activity of 98.5% from the certificate of analysis (COA) of this monomer was used. The assay spec of this monomer was relatively low (≥97%), in comparison with others.6
4. Other parameters:
- For simplicity of the experiments, no post additions (neutralizer and biocide) were made.
- Target solids: 45 ± 1%, Target conversion >97%.
- Initiator: ammonium persulfate at level = 0.1+0.4 = 0.5 pphm.
- Buffer: sodium bicarbonate at level = 0 or 0.3 pphm. Note that pphm = parts per hundred monomers.
- Defoamer: 0 or 0.1 pphm.
5. Experiment evaluation:
- Fouling;
- Grit level (ppm, milligrams of grit per Kg of latex);
- Solids ( by gravity method, avg. of triplicate);
- pH;
- Particle size (Zetatrac);
- Zeta potential (Zetatrac);
- Latex viscosity (RV #2@20).
Water Solubility of Monomers
There is currently no unified measuring system for the hydrophobicity of monomers. This property is normally compared based on the water solubility of monomers on reverse scale. However, the water solubility data of the hydrophobic monomers that have hydrocarbon chain longer than C8 are not readily available. Below are the water solubility data available in the literature.7,8
Data in Table 1 show that VeoVa 10 vinyl ester is more than 10 times less water soluble than 2-EHA, or in other words, VeoVa 10 monomer is more than 10 times more hydrophobic than 2-EHA. In practice, VeoVa 10 monomer is used in EP at levels ≤30% in vinyl acetate/VeoVa 10 copolymers or as modifier for acrylic polymers.
In general, the hydrocarbon chain length, the position and the bulkiness of the side group are considered contributing to the hydrophobicity of the monomer. We assumed that the hydrophobicity of IDMA is similar to that of VeoVa 10 monomer.
Inhibitor Levels of Hydrophobic Monomers
Because the monomers are used without purification, high inhibitor levels in the monomers is a concern. Table 2 shows typical inhibitor levels reported in different monomers.
Most of the hydrophobic monomers contain 100 ppm or higher inhibitor levels, while common monomers in current EP systems have inhibitor levels ≤30 ppm. Among the hydrophobic monomers, VeoVa 10 monomer has the lowest inhibitor level of 5 ppm monomethyl ether of hydroquinone (MEHQ). Tert-butyl methacrylate (TBMA) has 200 ppm MEHQ, the highest inhibitor level. Except VeoVa 10 is currently used at low levels in EP, others are not. Once the utilization of hydrophobic monomers is successfully proven in the emulsion polymerization industry, the reduction of inhibitor level might become a real need.
Results and Discussion
In this section, we first demonstrate the suitability of DPOS surfactants in the emulsion polymerization of hydrophobic monomers including VeoVa 10 monomer and IDMA, and second the potential applicability of this hydrophobic waterborne polymer technology to a wide range of applications through copolymerization and terpolymerization.
The compositions of copolymers or terpolymers were devised in a way so that the glass transition temperature (Tg) of resulting products would have values in the range of 13 °C-15 °C, as calculated by Fox equation. Therefore, all resulting latexes are expected to form film at room temperature.
In this article we will not discuss the zeta potential and latex viscosity for all data in the tables. However, based on our experience:
- Zeta potentials of the anionic latexes in the range of -50±5 mV would have adequate shear stability, without excessive foaming.
- Adequate mixing in the reactor was observed with latexes that have un-neutralized viscosities lower than 300 cP measured at #2@ 20 rpm.
VeoVa 10 Homopolymerization
Results of VeoVa 10 vinyl ester polymerizations using three 3 DPOS surfactants differentiated by alkyl group as described earlier are summarized in Table 3.
Overall, VeoVa 10 homopolymerization in this work resulted in excellent final conversion (>98.5%). With the use of C15 branched DPOS surfactant (experimental product) and C16 linear DPOS surfactant (Calfax 16 L-35), VeoVa 10 homopolymerization resulted in excellent in-process stability with low grit levels. C12 branched DPOS surfactant (Calfax DB-45) resulted in “slight-medium” fouling, although it performed well for current EP systems.
The results indicated that for homopolymerization of VeoVa 10 monomer, the longer the alkyl chain of DPOS surfactant, the better the effectiveness of surfactant in terms of:
- particle size efficiency (ability of generating smaller particle size at same surfactant level); and
- reactor cleanliness and low grit level.
Among three DPOS surfactants investigated, C16 linear DPOS surfactant (Calfax 16L-35) offered the best EP process performance for VeoVa 10 monomer. This surfactant was then selected as the primary surfactant for continuing studies of hydrophobic monomers.
Isodecyl Methacrylate Homopolymerization
Results of the homopolymerization of isodecyl methacrylate are summarized in Tables 4 and 5. We found a number of interesting and important results for the homopolymerization of IDMA:
- The use of buffer (NaHCO3) resulted in the pink color of the latex, while no pink color was observed with VeoVa 10 monomer.
- At the same surfactant level and split, IDMA resulted in smaller latex particle size than that of VeoVa 10 monomer.
- At the same surfactant level and split, the use of buffer resulted in higher conversion than without buffer.
- Buffer seemed to act as a defoamer in the preparation of pre-emulsion, preventing the introduction of foam into the reactor.
- IDMA tended to generate more foam in the pre-emulsion than VeoVa 10 monomer.
- Without the use of buffer, defoamer was necessary to break the foam.
- Poor emulsification was observed at 300 rpm mixing speed in the preparation of the pre-emulsion, and this tended to lower the latex conversion.
In the homopolymerization of IDMA without buffer we noticed that the foam was introduced to the reactor and foaming was likely the cause for the reactor wall fouling; and small latex particle size and high latex viscosity was likely the cause for the agitator fouling. We were able to fix the issue by the above observation and establish a formula without buffer (Table 5) that resulted in a latex with excellent in-process stability and final conversion of 97-97.5%, as shown in Table 5. The lower conversion of IDMA homopolymerization, in comparison with VeoVa 10 vinyl ester, is likely due to the high inhibitor content in the monomer and lack of buffer.
Copolymerization of VeoVa 10 Monomer and TBMA
Results of the copolymerization of VeoVa 10 monomer and TBMA are summarized in Table 6. The first three polymerizations of VeoVa 10 vinyl ester/TBMA =80/20 shown in Table 6 were made with 0.3 pphm NaHCO3. These polymerizations resulted in stable latexes with good in-process stability, good conversion and particle sizes of about 85 nm. However, these latexes showed a light pink color. Although the pink color was not observed in the dry polymer film, the color of the latex might give an unfavorable perception to downstream users. The light pink color of the copolymer latex may have come from the interaction of TBMA and NaHCO3 because the VeoVa 10 homopolymer latex containing NaHCO3 did not have such color. When NaHCO3 was reduced or eliminated from the copolymer formula we observed significant reduction in latex conversion. We thus speculated that the pink color might be the result of an acid-base interaction between MEHQ and NaHCO3, but no attempts were made to understand this behavior. Systematic experiments with chemical analysis would be necessary to provide better insights to this behavior.
Incorporation of 2.0 pphm of MMA or AA into the copolymer of VeoVa 10 vinyl ester and TBMA with the use of 0.3 pphm NaHCO3 in the formula resulted in no pink latexes. These latexes were acceptable on both processing and latex properties; with latex particle sizes in the vicinity of 100 nm. The latexes functionalized with MAA showed higher latex pH than AA counterpart, indicating more MMA buried in the polymer. However, the earlier latexes showed slightly better in-process stability and conversion. This might indicate that MAA is more compatible with this particular monomer composition.
Copolymerization of VeoVa 10 Monomer and MMA
Results of the copolymerization of VeoVa 10 monomer and MMA are summarized in Table 7. As shown in this table, the use of MMA in the copolymerization with VeoVa 10 vinyl ester significantly enhanced the latex conversion. The benefit of NaHCO3 on conversion was significant with this copolymer composition. We noticed very slight pink color exhibited with the presence of NaHCO3, but this may or may not be a concern in practice. Incorporation of 2.0 pphm MAA or AA into this copolymer composition resulted in good processing latexes. No significant differences were seen between MAA and AA in the processing of the latexes, however, AA resulted in easier filtration, slightly lower latex pH and slightly lower latex conversion. All functionalized latexes with acid monomer did not show any trace of pink color when in the presence of NaHCO3.
Terpolymerization of VeoVa 10 Monomer, TBMA and MMA
Results of terpolymerization of VeoVa 10 monomer, TBMA and MMA are summarized in Table 8. At the time of writing this article, we were not yet able to conduct the repeat runs for this and some other compositions. The results in Table 8 show that all the terpolymers made were excellent on both processing and latex properties. All the latexes showed no pink color, which is consistent with the findings observed and discussed earlier.
Copolymerization of IDMA and MMA
Results of copolymerization of IDMA and MMA are summarized in Table 9. In general, incorporation of MMA with IDMA significantly improved the latex conversion, in comparison with that of IDMA homopolymerization. The effect of NaHCO3 on latex color and conversion was again observed with this copolymerization system, due to the presence of IDMA. With a number of attempts shown here, we were not able to make latexes with good processing without acid functionalization and without the use of NaHCO3. Copolymers with acid functionalization and use of NaHCO3 resulted in latexes with good processing and properties. In this case, AA gave slightly better in-process stability and smaller particle sizes. All of the latexes made with this copolymer composition showed easy filtration, regardless of the fouling of the reactor.
Copolymerization of IDMA and Styrene
Results of copolymerization IDMA with styrene are summarized in Table 10. In general, incorporation of styrene with IDMA significantly improved the latex conversion, in comparison with that of IDMA homopolymerization; although the improvement was slightly less than the case of MMA. It was interesting to note that no pink color was observed with the use of NaHCO3, even in the case of no functionalization.
Copolymerization of IDMA and TBMA
Results of copolymerization of IDMA and TBMA are summarized in Table 11. At the time of writing this article we have just started the work with this copolymerization. Initial results indicate the composition of IDMA/TBMA = 50/50 resulted in fairly low conversion and poor fouling. This was likely contributed by the low reactivity of TBMA. We have made a couple of homopolymerizations of TBMA and these resulted in significantly low conversions of about 85%. The fouling was even worse and rated as med-heavy. The presence of NaHCO3 resulted in a light pink color, which is consistent with earlier results. Both IDMA and TBMA provided the source for the color formation. This color issue can be circumvented by either removing the buffer or adding a small amount of acid monomer to compositions containing buffer. Due to much lower reactivity of TBMA in comparison with other monomers, limited content of TBMA in the co- or terpolymerizations may be necessary to maintain reasonable conversion.
Conclusions
High conversion and stable latexes of VeoVa 10 monomer and IDMA made in this study show that diphenyl oxide disulfonate surfactants can be used to efficiently polymerize hydrophobic monomers. Ammonium persulfate was an effective initiator for these emulsion polymerizations. Copolymerization or terpolymerization of VeoVa 10 monomer and IDMA with other monomers such as MMA, styrene, TBMA have shown promising results.
Findings regarding the impacts of buffer on the color of the latex, latex conversion and the defoaming of the pre-emulsion, the interaction of acid monomers and styrene with buffer, as well as the acceleration effect of MMA and styrene to IDMA offered useful tools in designing effective copolymers and terpolymers of VeoVa 10 vinyl ester or IDMA with other monomers.
Using DPOS surfactants in emulsion polymerization of hydrophobic monomers may be a path to replace solventborne coatings and open up markets and applications for waterborne coatings, particularly on metals or on exterior surfaces. n
References
1 Avramidis, K.S.; Bassett, D.R. Emulsion Polymerization of Hydrophobic Monomers, US 2009/0264585 A1, Oct. 22, 2009, The Dow Chemical Co.
2 Zhan, Z.; Cheng, Q.; Wang, H. Process for Emulsion Polymerizing Hydrophobic Monomers, WO 201375293 A1, May 30, 2013, Evonik Industries AG.
3 Shabnam, R.; Ali, A.M.I.; Miah, M.A.J.; Tauer, K.; Ahmad, H. Influence of the Third Monomer on Lauryl Methacrylate-Methyl Methacrylate Emulsion Terpolymerization, Colloid Polym. Sci. (2013) 291: 2111-2120.
4 Fontenot, K.J.; Schork, J.; Reimers, J.L. Miniemulsion Polymerization Process Using Polymeric Co-Surfactant, US 5,686,518, Nov. 11, 1997, Georgia Tech.
5 Lau, W. Method for Forming Polymers, US 5,521,266, May 28, 1996, Rohm and Haas Co.
6 Certificate of Analysis of Bisomer IDMA, Material No.: 745805, Batch No.: 1750604011.
7 Vanaken, D. High-Performance Water-Repellent Acrylic Latices for Exterior Wood Coatings, Momentive Brochure.
8 Handbook of Detergent, Part D: Formulation, Edited by Michael Showell, CRC Press, Jul 27, 2005, page 397.
For more information, e-mail bdnguyen@pilotchemical.com. To contact Pilot Chemical Company regarding products and services, visit www.pilotchemical.com or call 1 (800) 70-PILOT.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!






















