Coatings’ Cobalt Replacement Challenge
Where Are We Now?

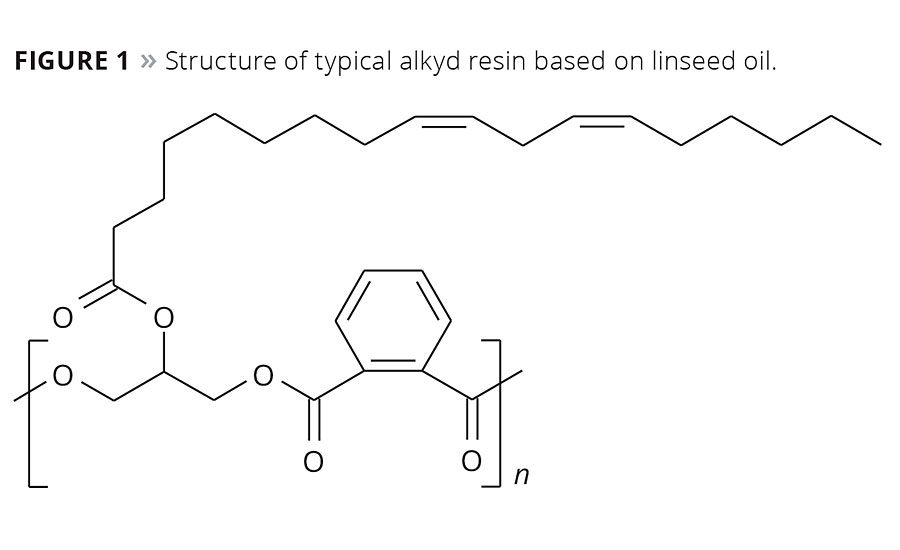
Figure 1

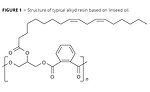
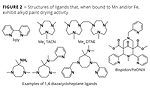
Figure 2

Cobalt (Co) soaps, or more accurately, cobalt carboxylates, is the collective description for a range of partially soluble compounds formed by cobalt in combination with a range of organic acids. These typically have a carbon chain with between 8 and 10 carbon atoms; the most common being ethylhexanoate (C8), isononanoate (C9) and neodecanoate (C10) or, in selected applications, natural naphthenates.
These substances are used as a primary drier or siccative in alkyd resin-based paints that make up about 40% of the market and are used widely for both decorative and functional end uses. An example of a typical linseed oil-based alkyd resin is given in Figure 1. The coatings sector today is well versed in the regulatory issue that cobalt has come to present. Working in close co-operation with paint makers, research and development groups have found some solutions, and a number of paint makers already make claim to being “cobalt-free,” but how did we get there, where are we now and how do these developments inform the next step?
Concerns with Cobalt
The use of cobalt became widespread following the removal of lead in paints. However, there has been a long-running debate in industry about the issue of bioavailability of cobalt and read-across.
The European Chemicals Agency (ECHA) is considering several cobalt compounds for authorization under REACH, and the Cobalt Reach Consortium chose to self-classify 12 cobalt substances under the EU CLP Regulation as Toxic to Reproduction Category 2: more commonly referred to as CMR 2. This wider list now includes a range of commonly used carboxylates.
The Dutch Authorities have informed ECHA of their intention to submit CLH (harmonized classification and labeling) dossiers for cobalt and cobalt compounds for classification as carcinogenic 1B and reprotoxic 1B.
Alongside these regulatory moves in Europe, the National Toxicology Program in the United States has written several reports on cobalt, and in June 2015 issued a draft report concluding that cobalt and certain cobalt compounds are reasonably anticipated to be human carcinogens.
With the reclassification of cobalt looking likely, there is now added impetus on the coatings sector to continue the search for alternatives that can deliver comparable drying performance to cobalt, while working in harmony with other technical additives. The team behind Catexel was the first to develop and market, via OM Group in the United States, a viable cobalt replacement product with its iron-based FeONIX technology, and the chemistry behind this important breakthrough is discussed later in this article. The team has since been harnessing the full range of its iron- and manganese-based platforms with the proven potential to present long-term solutions that address the paint manufacturers’ overriding concerns about maintaining performance without compromise and while remaining cost effective.
A Catalyst for Change
The search for a cobalt substitute has not been an easy one, and there is no such thing as a simple drop-in cobalt-free solution. The universally useful cobalt soaps leave a big hole to fill and one that needs a range of optimized solutions.
In particular, any viable alternative must be:
- applicable in all alkyd-based (solvent-based, water-based and high solids) coating segments, and in all geographies;
- fully compatible with other materials used in paints and maintain the aesthetic, protective and functional properties of the coating;
- environmentally sustainable;
- cost effective.
While posing a troublesome challenge for manufacturers, the regulatory threat jeopardizing cobalt’s long-term viability does provide an opportunity for the makers of paints, inks and composites to consider innovative technologies.
In this search, a variety of substitute siccatives have been explored, in particular platforms based on manganese (Mn) or iron (Fe) either as a complex or as a redox-active transition-metal salt combined with a ligand.
Manganese Improves Drying Performance
Traditional manganese soaps, while more active than iron, are still less active than cobalt soaps. What is more, the presence of Mn(III) in commercial manganese soap solutions makes the substance intensely colored, posing a challenge for paint manufacturers. To counteract this, in many cases, the traditional Mn soaps still need to be formulated with cobalt soaps to reduce the levels of both metals. Alternatively, they need to be activated by the inclusion of a ligand.
By choosing an appropriate ligand, which upon binding to the metal ion tunes the properties in such a way that the autoxidation reactivity within the alkyd resin is enhanced, the level of active Mn-based drier can be reduced.
As Figure 2 demonstrates, a number of nitrogen-based ligand donors have shown to improve the paint drying activity of Mn salts significantly, leading to potential alternatives to cobalt soaps.
It should be noted, however, that alongside paint drying times and color of the metal drier, other parameters still need to be fully explored and understood to make a proper assessment of the impact of a new paint drying catalyst. These parameters include: the stability in the paint formulation (due to decomposition of the active drier or adsorption on pigments or fillers), color formation of the paint layer upon ageing (yellowing) and hardness development of the paint layer. In fact, a more complete overview of Mn, as well as Fe, paint drying catalysts can be found in a recent review.1
The addition of 2,2’-bipyridine (bpy) (Figure 2) is one avenue under exploration. Adding bpy leads to improved drying activity of Mn soaps and is therefore applied at levels of Mn that are similar to those used for cobalt. Despite its improved drying capabilities, however, the solution is still colored to some extent, which does limit its applicability. Recent detailed investigations have revealed that upon mixing Mn soaps and bpy, multinuclear complexes may be formed, and these could be involved in the paint drying activity.2
Manganese complexes using 1,4,7-trimethyl-1,4,7-triazacyclononane (Me3TACN, Figure 2) and 1,2-bis(4,7-dimethyl-1,4,7-triazacyclononan-1-yl)-ethane (Me4DTNE, Figure 2) as ligands have also been studied as well-defined complexes and as mixtures of manganese salts. The manganese complexes or mixtures with triazacyclononane-based ligands have received considerable attention as alkyd paint drying catalysts. Following is a summary of different publications and patent applications.
[MnIV2(m-O)3(Me3TACN)2](PF6)2 was studied in model paint formulations and more extensively on model substrates such as ethyllinoleate at Eindhoven University.3 The paint drying activity, compared to Mn soaps, was clearly improved, and the Mn level’s ability to furnish good drying activity was similar to that of the cobalt level under the same conditions. Activity in both solventborne and waterborne paints was observed. Improvement of the drying activity was highlighted when using other Mn-Me3TACN complexes, such as [MnIII2(m-O)(m-RCOO)2(Me3TACN)2]2+ and [MnIV2(m-O)3(Me3TACN)2](CH3COO)2 complexes.4,5 Also improvements in the drying performance were recorded as a result of mixing ligand and manganese salts.6,7,8 Combining [MnIV2(m-O)3(Me3TACN)2]2+ and Me3TACN can lead to enhanced paint drying activity, thereby lowering the concentration of Mn required in the paint formulation.9
Other studies show that manganese complexes with the bridged TACN ligand, Me4DTNE, actively aid paint drying too.10 Further research has highlighted that mixtures of ligand and manganese salts are much more active than the well-defined [MnIVMnIII(m-O)2(m-H3CCOO)(Me4DTNE)]Cl2 complex.
Similarly, formulating Me3TACN, Me4DTNE or bpy ligands into the alkyd resin (without manganese) can lead to storage-stable mixtures that upon addition of manganese salts show paint drying activity.11
Diazacycloheptane-based ligands – a new class of ligands – are being explored as alternative catalysts with Mn. The advantage of these is that by using different building blocks, a variety of the diazacycloheptane-based ligands can be obtained, each having slightly different properties. This in turn tunes the paint drying activity, but may also change other properties of the siccatives. Further research into other classes of manganese catalysts is ongoing and will be published in due course.12 Examples of other Mn complexes have been reported to be active for paint drying and these are discussed in a recent review paper.1
Iron Based Solutions
Alongside manganese, iron-based alternatives are showing further potential for development. Traditional iron soaps have much lower reactivity towards alkyd resin curing than cobalt soaps and, like manganese, are more intensely colored. As a result, they have been put to more limited use, especially in high-temperature curing – for example in stoving enamels.
Iron catalysts are also being investigated, and while being generally less active than Fe soaps, some show a very good paint drying activity. An example of an active iron complex can be found in Figure 2. Here, the ligand structure is a pentadentate nitrogen ligand based on bispidon. This iron-bispidon catalyst, which is the aforementioned FeONIX technology, was originally developed for food-oil stain removal activity in detergents, and it was shown that autoxidation radical chemistry reactions are involved in the removal of such stains.13
Following on from this work, other studies discovered that the autoxidation radical chemistry operative for food oil stain bleaching is very similar to that of alkyd paint hardening catalyzed by cobalt soap siccatives. Initial experiments were carried out using linseed oil as an indicator for activity in paint and ink formulations, and good drying activity was observed with it and other catalysts.14
Subsequently, an iron bispidon catalyst was further developed for this application. More details on the reactivity and paint drying activity of this Catexel catalyst, to which OM Group currently holds the IP rights for use in inks, coatings and composites and markets under the Borchi Oxy Coat banner, can be found in Reference 14.
Alternative iron-based catalysts for more widespread use are also being explored, namely those based on diazacycloheptane ligands (Figure 2),15 and have shown improved paint drying activity (compared to cobalt soaps and Fe soaps). A recent publication discusses these and other iron catalysts in more detail.1
Looking to the Future
With the reclassification of cobalt-based alkyd curing catalysts highly likely, research is ongoing to find transition metal ion alternatives that also boast improved safety and environmental profiles.
While in the last two decades Mn and Fe catalysts have been found to offer higher drying activity than cobalt, manganese and iron soaps, other parameters are still yet to be explored that may impact the formulation of coatings in the future. As paint compositions have previously been optimized for cobalt soaps, manufacturers may need to make some adaptions to ensure new Mn or Fe siccatives work in the most optimal manner.
While keeping these aspects in mind, the search for metal complexes based on manganese and iron, and the accelerating effect of polydentate ligands on existing driers has led to the discovery and development of multiple product platforms able to meet the varying needs of the paint maker. Catexel is at the forefront of this discovery and development, and is helping industry to make the change from cobalt soaps to manganese and iron driers, as well as also looking to the future with next-generation technologies under development.
With more viable alternatives very much on the horizon, paint manufacturers will be looking for a range of solutions that not only replace, but in many aspects seek to improve on the performance of cobalt and offer low-dose, highly selective products that do not experience the high price volatility and geopolitical issues experienced by cobalt.
Now and in the future, paint makers can look to innovative partners to tailor molecules for any and all applications as part of the everyday process of improving the aesthetics, performance and environmental profile of coatings in general and paint in particular.
References
1 de Boer, J.W.; Maaijen, K.; Hage, R. Manuscript in publication (2016).
2 van Gorkum, R.; Bouwman, E. Coord. Chem. Rev., 249, 1709 (2005).
3 Oyman, Z.O.; Ming, W.; van der Linde, R.; ter Borg, J.; Bieleman, J.H. Surf. Coatings. Int. Part B, Coatings Transactions, 88, 269 (2005).
4 Jansen, J.; Kleuskens, E.C.; van Summeren, R.; Alsters, P.L. EP2534215B.
5 Jansen, J.; Bergman, F.A.C.; Kleuskens, E.C.; Hage, R. WO2011/098584.
6 Meijer, M.D.; van Weelde, E.; van Dijk, J.T.M.; Flapper, J. WO2013/092441.
7 Meijer, M.D.; van Weelde, E.; van Dijk, J.T.M.; Flapper, J. EP2794787B.
8 de Boer, J.W.; Maaijen, K.; Hage, R. WO2015/114349.
9 Meijer, M.D.; Flapper, J. WO2014/095670.
10 de Boer, J.W.; Maaijen, K.; Hage, R. WO2014/122432.
11 de Boer, J.W.; Maaijen, K.; Hage, R. WO2014/122433.
12 Maaijen, K.; Hage, R. patent filed on new Mn-based siccatives.
13 de Boer, J.W.; Wesenhagen, P.V.; Wenker, E.C.M.; Maaijen, K.; Gol, F.; Gibbs, H.; Hage, R. Eur. J. Inorg.Chem., 3581-3591 (2013).
14 Hage, R.; Wesenhagen, P.V. WO2008/003652.
15 de Boer, J.W.; Maaijen, K.; Hage, R. WO2014/122434.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!









