Solving Film Defects with Surface Tension Modifiers
Taking the Mystery Out of “Pixie Dust”










Formulators face many challenges when developing new products or supporting older product lines. Film defects like air entrapment, crater formation, poor flow and leveling, substrate wetting, pinholes and crawling are just some of the problems encountered after the binders and pigments have been optimized. The term “pixie dust” is given to a mystical chemical that when added to a coating will fix one or more of the above problems, but the exact mechanism is not known. Chemists have long relied on the trial and error method of tweaking their formulas with a myriad of additives in the hope that one or more will work. The available information about these additives is usually veiled in secrecy or labeled with generic chemical names. In this article we will provide information about identifying the root causes of some of these film defects, how additive manufacturers have developed strategies to modify the surface tension of liquid coatings, and how the formulator can make intelligent choices and thereby reduce the number of products to test.
The Source of Many Coatings Defects
Most coatings are supplied or applied in a liquid state, and all liquids obey certain laws of nature. The characteristic of liquids that specifically causes the film defects previously mentioned is their tendency to retain the smallest shape possible (which is a sphere) due to molecular cohesion. This cohesive force is more commonly known as surface tension and is measured in dynes/centimeter. The defects arise when the liquid coating comes in contact with other objects that have different surface tension values such as solid surfaces, pigments, other liquids and even entrapped air. Solid materials are typically characterized by surface energy, which is their tendency to attract other materials measured in dynes/centimeter.
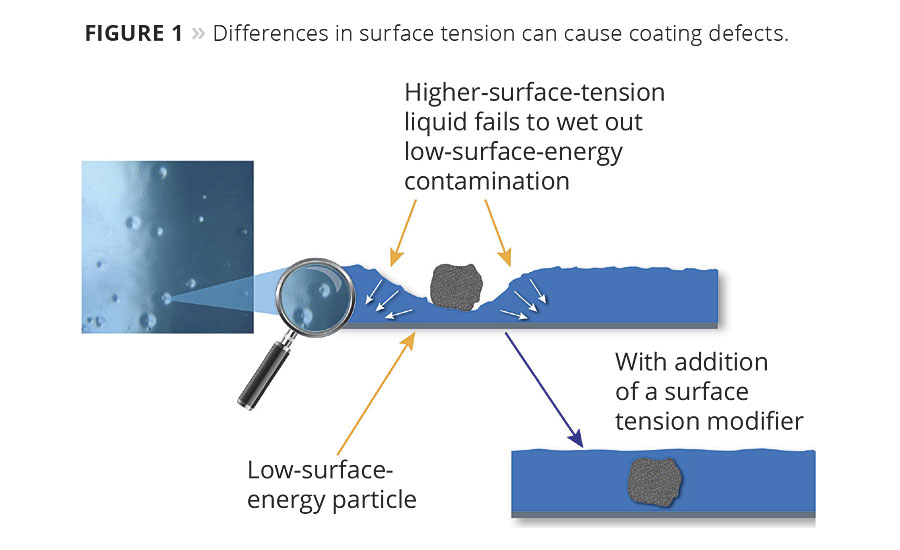
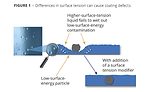
In the case of substrate wetting, the surface tension of a liquid coating must be less than the surface energy of the substrate to which it is being applied; otherwise it will not form a continuous or smooth film. The same is true for materials that contaminate a coating. The surface tension of the coating must be less than the surface energy or surface tension of the other materials; otherwise the coating will draw away from them, resulting in crawling or crater formation (Figure 1).
For example, in Table 1 we see that xylene would easily wet out any of the substrates listed because its surface tension value of 29 is lower than the other surface energies. However, water would tend to form beads on most of these materials because its surface tension value of 72 is higher than most surfaces except for glass.
The equation here is used to determine the minimum film thickness that a liquid needs to overcome surface tension issues over a substrate.
Where:
- h is the minimum depth of liquid in centimeters;
- ¡ is the surface tension of the liquid in dynes per centimeter;
- g is the gravitational constant (980 cm/s2);
- r is the density of the liquid in grams per cubic centimeter.
Mercury has a very high surface tension and will not wet out many surfaces. However, if you pour enough mercury into a glass jar it will completely cover the bottom because the depth of the liquid exceeds mercury’s surface tension and will form a continuous layer. This is impractical in coatings applications where costs and physical properties limit the amount of coating being applied. The only reasonable option is to lower the surface tension of the coating to values less than the surfaces, liquids and contaminants it is likely to come in contact with.
Additives that Reduce the Surface Tension of Liquids
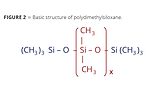
There are a variety of techniques to lower surface tension and they depend in part upon the composition of the coating – whether it is water- or solvent-based and, if solvent-based, if the solvent blend is polar or nonpolar. The most practical methods employ using low-surface-tension chemicals such as solutions of hydrocarbons or polydimethylsiloxanes (PDMS). Hydrocarbons can consist of organic compounds like xylene, mineral spirits or mineral oils. However, they are mainly effective in water-based systems to help remove air from coatings during the manufacturing or application processes. Polydimethylsiloxanes are chemical compounds that can be modified in a variety of ways to address specific defect issues caused by surface tension. The basic structure of PDMS-based additives is found in Figure 2.
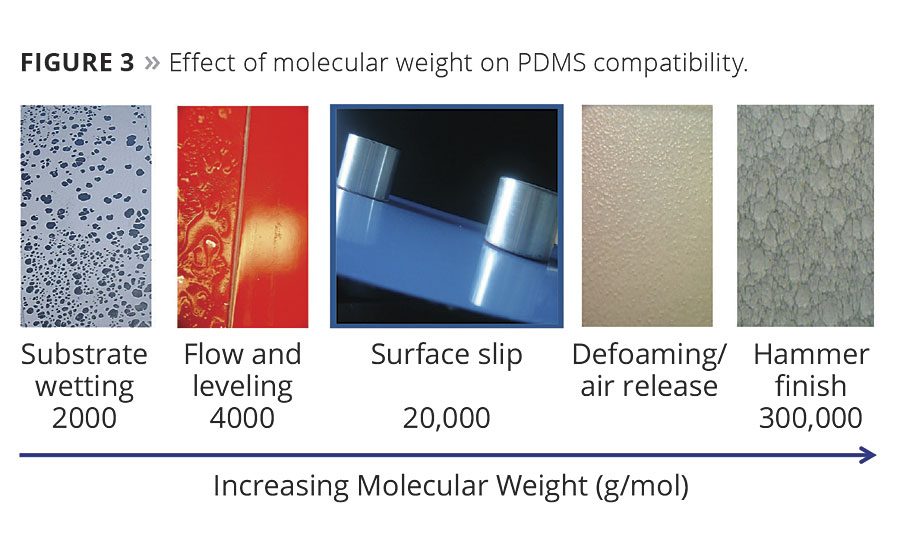
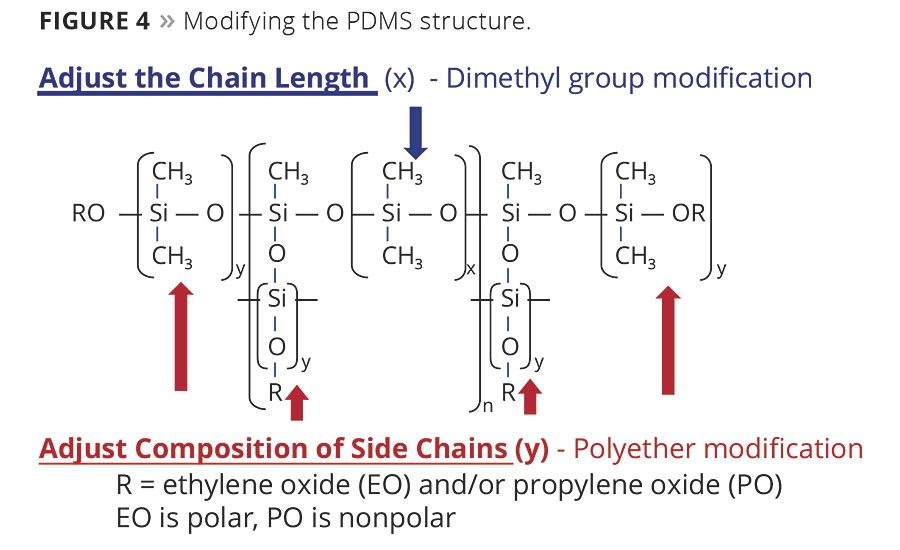
Increasing the number of siloxane units (X) will increase the molecular weight of the additive and alter its compatibility in a solvent medium. As seen in Figure 3, low-molecular-weight PDMS-based additives tend to be more compatible and can improve wetting and flow, where extremely high-molecular-weight materials can be so incompatible that they can only be useful in generating specialty finishes. However, compatibility and solubility can be more finely controlled by adding specific amounts of organic components or polyethers, as seen in Figure 4.
Adding components like ethylene oxide (EO) or propylene oxide (PO) will determine the solubility of the polysiloxane in liquid mediums. Adding more PO than EO will improve the compatibility of the additive in nonpolar solvents like aromatic or aliphatic hydrocarbons. Adding more EO than PO will improve the additive’s compatibility in polar solvents like water, alcohols and ketones. How these organic components are attached will also determine the additive’s long-term stability in water. If they are attached via ether linkages (i.e. butanol initiated) they can show instability in water-based coatings and should only be used in systems based on organic solvents. If attached directly to the polysiloxane backbone via allyl-initiated polyethers they can be 100% active and used in both water- and solvent-based systems.
High-Temperature Applications
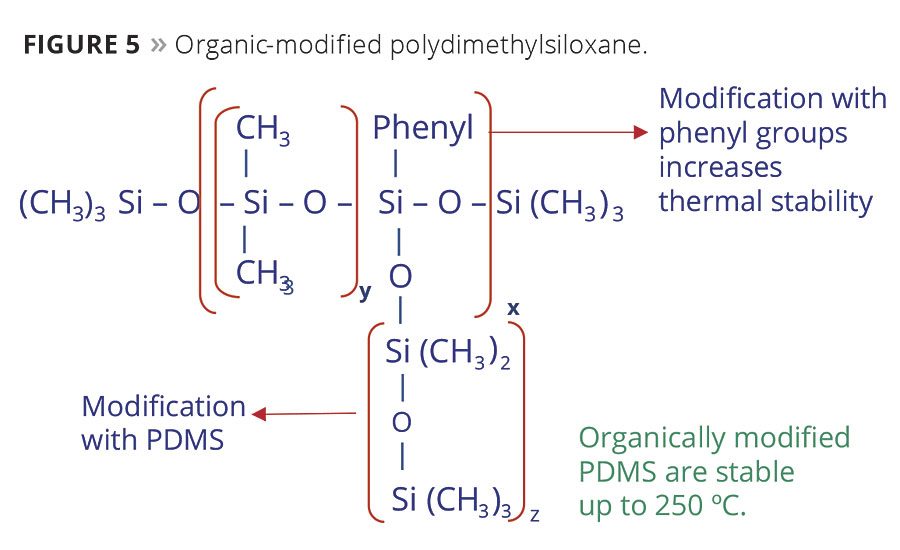
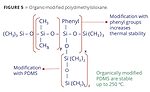
Thermoset applications like packaging and coil coatings require heat-stable additives. Polydimethylsiloxane can be modified with phenyl groups or other PDMS side chains to increase their decomposition temperature (Figure 5). Additives with these structures are stable to 250-300 °C, while unmodified versions will decompose at 200 °C.
Multiple Benefits
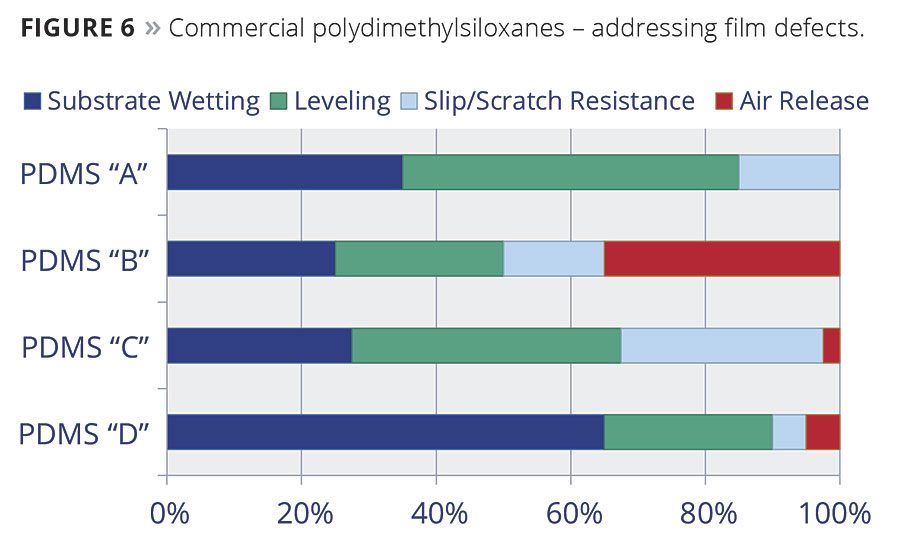
Coatings systems contain various mixtures of solvents, pigments and binders whose blend of surface tension materials can cause the film defects mentioned earlier. As such, the compositions of commercial PDMS-based additives vary to address these defects in the widest range of coatings possible. Molecular weight, polarity and carrier solvents (if any) will determine how effective the commercial PDMS is in specific coatings systems, but they may also provide benefits to other properties. Figure 6 shows the different properties that several PDMS-based additives can have on coatings.
For example, additives that are primarily effective for substrate wetting and/or leveling (A and D) tend to have relatively low molecular weights, while those with higher molecular weights (B and C) can exhibit a range of benefits, including air release.
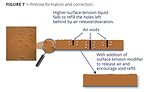
An example of this would be to correct pinhole formation – a common defect that occurs when air is entrapped in a thick film and slowly releases, leaving a void behind, sometimes down to the substrate. The void or “hole” does not refill fast enough before the coating begins to solidify. To correct this, a multifunctional PDMS is required that must encourage air release and also provide improved flow and leveling before the coating begins to set (Figure 7). In this case, PDMS “B” would be the product of choice.
Summary
Many film-related defects are caused by the liquid coating’s surface tension as it interacts with other materials that have low surface energy. These “materials” can be in the form of air, other liquids and solids encountered during the manufacturing or application process, and/or the surface to which the coating is being applied. In practical situations, the surface tension of liquids can only be reduced with the addition of low-surface-tension modifiers such as hydrocarbon blends and additives based on polydimethylsiloxane. PDMS-based additives can be modified by increasing their molecular weight and/or by designing the appropriate functionality to affect their heat stability, compatibility and/or solubility in various solvent systems. Focusing on using the right type of additive to address a specific film defect will reduce the number of materials to test and not rely on trying many different chemicals in the hope of finding just the right pixie dust.
For more information, visit www.borchers.com.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!