Performance Breakthroughs in Zinc-Free Floor Finish Composition












Across multiple industries, the poor performance of many first-generation environmentally preferred products created a belief that “going green” meant settling for reduced product performance. In floor care, the performance of zinc-free products supported this impression, especially compared to tried-and-true zinc-based technology. Now, a recent polymer innovation closes the performance gap in zinc-free offerings. This article provides a look at the development of zinc-free finishes and how they are finally ready to shine.
Floor Care Product Evolution
Floor finishes have evolved over the ages. For example, the use of beeswax to create a mirror-like finish on the floors of the French royal palace of Versailles is well documented. As the practice of floor maintenance evolved, carnauba wax became the standard. The result was the characteristic wax-based shine with the hallmark swirls created by the buffing process.
The birth of polymer science led to the development of the first synthetic floor finishes based on styrene-acrylic polymers. This timely evolution was welcomed because it was more efficient and affordable, especially given the cost of carnauba wax.
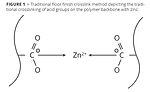
Such polymer-based floor finishes were unfortunately not without shortcomings: there was no easy removal method when replacing the finish. To improve removability, polymer chemists increased the acid value of the polymer so that a mild base could be used to break down the film and remove it from the floor. But, as the film was made more removable, it became more easily damaged, resulting in poor durability and performance. A simple yet creative idea was tested and proven to solve the removability problem: adding a divalent metal such as zinc to crosslink the pending acid groups of the polymer (Figure 1).
Metal crosslinking with zinc solved multiple issues. First, it enhanced the film’s durability by creating a harder, more scratch- and scuff-resistant film. Second, it solved the issue of water and detergent sensitivity, as the zinc tied up the reactive acid groups on the polymer. Third, zinc-based crosslinking ensured an easy means of removal because the polymer crosslinks would breakdown in the presence of an amine compound such as ammonia or monoethanolamine, allowing the film to be solvated in a liquid and removed.
For years, zinc-based floor finishes reigned supreme. Then, in support of the 1972 Clean Water Act, the EPA included zinc on a list of materials it considered “priority pollutants.” Among the materials on the list were those the EPA had determined to have a negative effect on water treatment and therefore should be kept from municipal wastewater streams. Zinc was included because it is an inhibitor of micro-organisms known to be beneficial to wastewater treatment.
While there are strong scientific arguments and studies that suggest that the zinc used in floor finishes remains bound to the polymer and does not cause negative environmental effects, organizations such as GreenSeal, Ecologo and U.S. EPA Safer Choice will certify floor care products only if they are zinc-free.
With zinc excluded from use in environmentally certified products, floor care chemists were once again challenged with creating a finish that maintained the hardness needed for scratch and abrasion resistance, and provided the necessary water and detergent resistance, yet was easily removed when the floor needed to be stripped and refinished.
The Development of Zinc-Free Floor Finishes
The first evolution of zinc-free floor finishes removed zinc and built molecular weight using specialty monomers frequently used in industrial coatings designed to internally crosslink the polymer chains during polymerization. These formulations were sometimes designed to create additional latent crosslinking after the film formed. While these types of polymers were an improvement over noncrosslinked polymers in terms of durability, they tended to be difficult to remove and were not very successful in the marketplace.
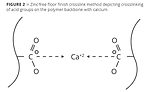
A second evolution of zinc-free floor finishes replaced the zinc with another divalent metal. While there are numerous metals on the periodic table from which to choose, many are more toxic than zinc. Others caused premature crosslinking and therefore had poor shelf stability. Ultimately, calcium was chosen as the replacement metal and has been the most common method of crosslinking for zinc-free floor finishes (Figure 2).
The fact remains, however, that neither of these technology evolutions perform as well as traditional zinc-based finishes. The method using specialty monomers to create strong internal and latent crosslinking improves durability, but is difficult to remove. The calcium crosslinked polymer technology offers easy removability, but falls short on durability. So there remained a need for an improved approach that achieves the necessary durability while also being easy to remove.
New Horizons
To overcome the challenge of providing a more environmentally friendly zinc-free floor finish while still achieving the level of all-around performance of traditional zinc-based finishes, it would be useful to review technologies in adjacent markets for clues and inspiration. For durability, mechanisms used in architectural coatings are well known. A number of these mechanisms build durability and hardness through combining the divalent crosslinking of calcium with very strong latent crosslinks. These include silane, epoxy, urethane and the like. But many of these methods come with side effects such as darker color, lower gloss or premature crosslinking from hydrolysis in water-based systems. Thus, achieving the overall performance of the crosslinking mechanism in traditional zinc-based finishes (Figure 1) required the development of a latent crosslink without these negative side effects that could be used in conjunction with calcium. The result was the development of a proprietary Twin-Linked™ crosslinking mechanism (Figure 3).
The new Mor-Glo®Ultra G floor finish polymer from OMNOVA Solutions features this proprietary Twin-Linked crosslinking mechanism. This product offers a step-change improvement in performance for zinc-free floor finishes. As shown in Tables 1-4, it provides performance equal to most zinc-based floor finish systems in terms of durability, with easy removal and maintenance. These performance data indicate that Twin-Linked polymers can be a stand-in for zinc-based products when environmental certification is a goal. The data even suggest that these polymers are a potential replacement for zinc-based technology altogether.
Tables 1-4 compare application and durability properties of various commercial floor finish systems. Included in these tests are commercially available zinc-free finishes, zinc-based finishes and a finish formulated with the new Mor-Glo Ultra G polymer. These lab studies were conducted using ASTM test methods or internally developed floor test methods developed to indicate application and durability of floor finishes.
Summary of Test Results
A floor finish that does not have good application properties is doomed to be a commercial failure. The finish must flow out on the surface, form to a clear film under various temperature and humidity conditions, be recoatable within 30 minutes of each application, and adhere well to the surface to which it is applied. Fortunately, all formulations performed well in the application tests (Table 1). It was in other areas that dramatic differences in performance were found.
The commercially available zinc-free floor finishes tested had low scores on durability compared to the zinc-based products and compared to the Mor-Glo Ultra G product that uses Twin-Linked technology (Table 2). In commercial use, such poor performance would likely lead to more frequent stripping and reapplication, thereby reducing the overall environmental benefits intended by a zinc-free finish. (This fact has led many floor maintenance contractors to discontinue use of zinc-free floor finishes unless required.)
Surprisingly, in testing of premium zinc-containing finishes, low durability scores in the areas of soil resistance, scuff resistance and water resistance were found.
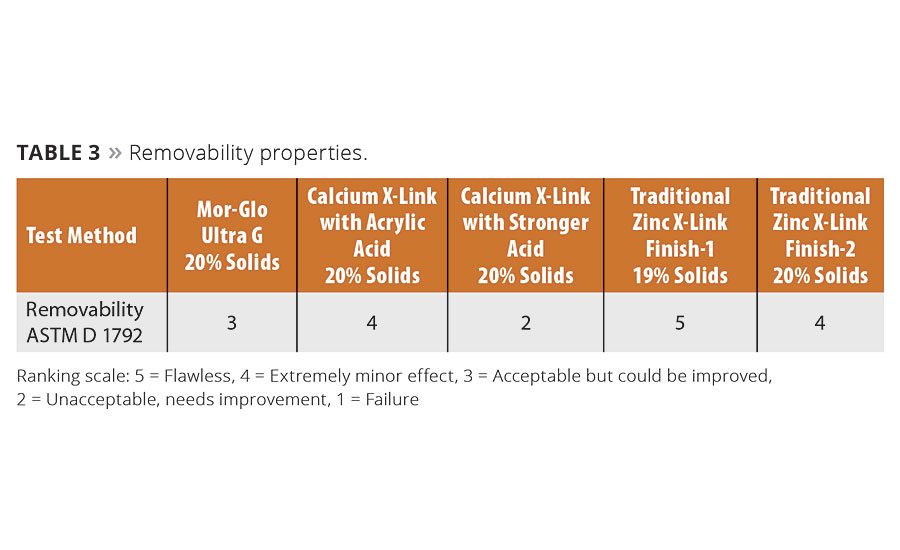
Removability remains a balancing act for zinc-free floor finishes. The test results show significant variability (Table 3), which likely stems from attempts to provide the right level of finish hardness and removability. Zinc-based polymers still perform best in this area. Finishes that employ polymers using the new Twin-Linked technology shows moderate removability, a measure that must be balanced against the durability and reparability of the finish.
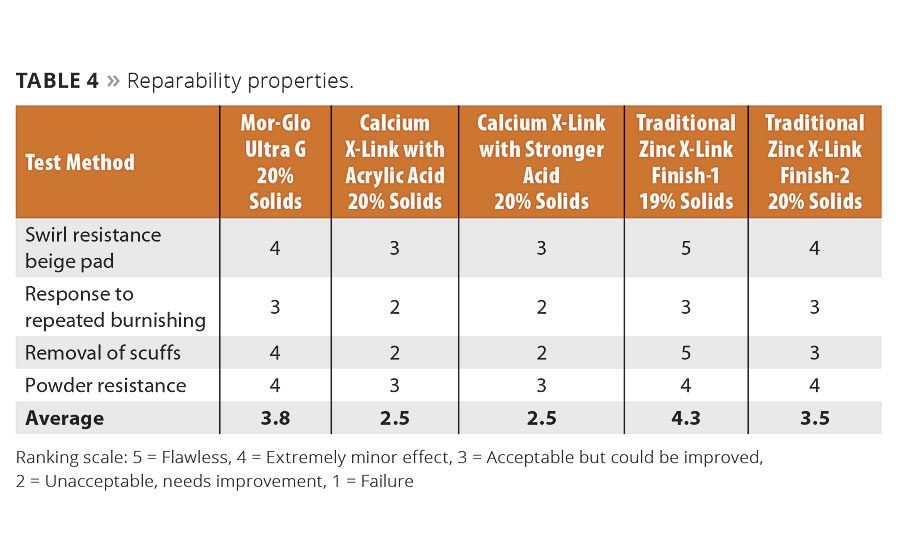
With good floor maintenance, which includes periodic burnishing to repair scratches and scuffs, a floor finish can last several years, even in moderately high traffic areas such as schools and industrial settings. To that end, the finish must exhibit good reparability with commonly used methods such as low-, medium- and high-speed burnishing. Early zinc-free finishes were challenged with high-speed burnishing because they were too soft and could not withstand the mechanical abrasion. They would swirl, powder and provide limited reparability, which led to premature stripping and reapplication. The new technology exhibits excellent reparability in testing (Table 4), exceeding that of the zinc-based products in some respects.
Conclusion
The evolution of floor care finish technology demonstrates that there is always a way to improve durability, removability and reparability. The most recent challenge has been to do so without using zinc as an ionic crosslinker. Omnova’s new Twin-Linked crosslinking innovation can now enable floor finish producers to develop effective solutions for customers needing products that have green certifications, while also offering the opportunity to extend the environmental benefits of zinc-free floor finishes to all users of traditional zinc-based floor finishes.
For more information, visit www.omnova.com.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!
















