Twisting Resin Properties
Polycaprolactones – a Versatile Tool to Modify Coatings Formulations

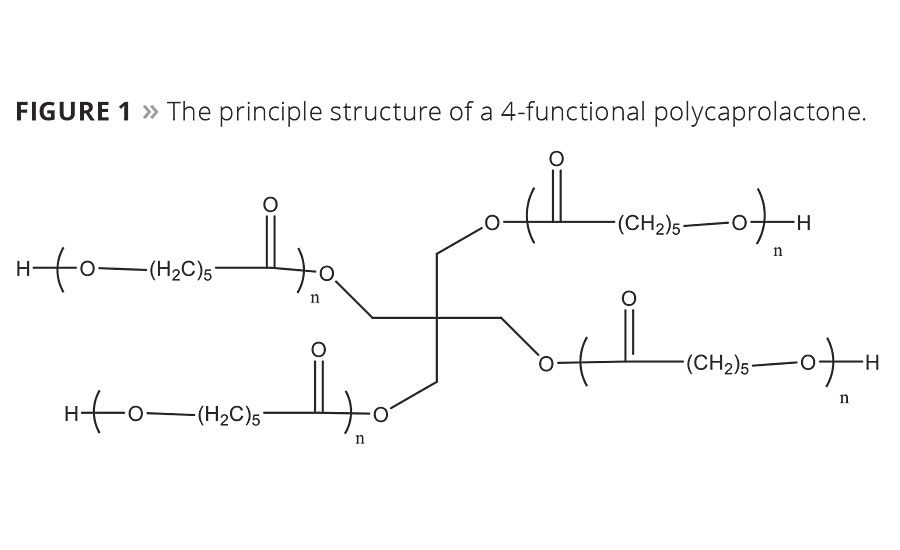














Polyurethane technologies are known to offer many technical advantages over competing coating systems. Two-component polyurethane (2K PUR) coatings especially are seeing widespread use in application areas like the automotive sector, windmills, aircraft, transportation, etc. Although it is a well-established technology, there is constant pressure to improve and fine-tune technical properties like abrasion resistance, scratch resistance and outdoor durability. Regulations also force formulators to further reduce VOCs in high-solid (HS) formulations or change to waterborne (WB) solutions. Very low-viscous caprolactones can be a tool to influence both VOC and technical properties by lowering emissions and prolonging the life cycles of coating systems – important criteria for improved sustainability.
Polycaprolactones
Perstorp has been producing polycaprolactones for 40 years and offers a range of polyols suitable for modifying and fine-tuning crosslinked coating formulations like 2K and 1K PUR systems. Properties of formulations based on conventional acrylics and polyesters can be tailor-made by the addition of polycaprolactone polyols.
Caprolactone chemistry offers a unique possibility to target specific requirements in different coating systems by utilizing a controlled ring-opening polymerization onto hydroxyl groups, resulting in a very low acid number (< 0.25 mg KOH/g). Semicrystalline polyols with a precise functionality and narrow molecular weight distribution can be prepared in a reproducible way. By choosing between different hydroxyl functional initiators, a versatile range of aliphatic, low-Tg (around -60 °C), solvent-free and low-viscous polyols can be selected when formulating high-performance crosslinked coatings for various applications.
With a range of Capa™ polyols varying in functionality of 2 to 4, OH content between 4.1-17% and molecular weight between 300-2000 g/mol it is possible to technically fine-tune coatings formulations. Normally a replacement between 5 and 20 wt% of standard polyols – acrylics or polyesters – will give a significant added value from caprolactones.
Polycaprolactones can be added as resin modifiers in solventborne (SB) systems based on acrylics or polyesters and various crosslinkers since they have a wide compatibility range. This makes the number of combinations almost unlimited. The fact that they are solvent free and are liquid at room temperature makes them suitable as reactive diluents for solvent-free systems. Grades with very low viscosities are also suitable to use in waterborne formulations based on dispersions where the low viscosity and relative high water acceptance caused by high hydroxyl numbers allow the polyols to migrate into the emulsion droplets.
This study shows the influence of adding different levels of 3-functional and 4-functional polycaprolactones (Figure 1) in HS 2K PUR systems. It also describes the principle of using them in waterborne emulsion-based systems.
Solventborne Formulations
Polycaprolactones are easily incorporated in solventborne formulations at any stage of paint preparation. Due to their excellent pigment wetting properties they should preferably be added during the grinding stage when used in pigmented formulations. In general, normal formulation principles are used when working with caprolactones, and conventional tools such as additives, solvent and crosslinkers are utilized when doing development work. Their well-defined molecular weight and functionality guarantee robust contributions to the final properties of the coating. Even if polycaprolactones by nature have a low Tg derived from aliphatic structures – and consequently less contribution to final hardness from physical drying – formulations can easily be made where hardness and mechanical properties are maintained or even increased. This is explained by increased crosslink density – a consequence of increased hydroxyl numbers – resulting in higher mechanical strength by strong hydrogen bonding between high amounts of formed urethane bonds. Gloss and haze are also maintained, or in many cases improved, due to perfect compatibility and improved flow derived from their low-viscous nature. Generally polycaprolactones have a high reactivity towards isocyanates due to their pendant primary hydroxyl groups at the end of flexible aliphatic chains. A desired balance between cure speed and pot life can be adjusted by the amount of catalysts added.
In this study, all formulations were based on a high-solids acrylic (Table 1). In formulations Capa A-C a conventional polyester polyol was replaced by a 3-functional polycaprolactone by different principles. In Capa A it was replaced on weight basis, in Capa B on basis of hydroxyl content and in Capa C the amount was doubled in order to further stretch influence on properties. In formulation Capa D the conventional polyester was replaced by a 4-functional polycaprolactone, on basis of hydroxyl content. The NCO/OH ratio was kept constant at 1.05.
VOC
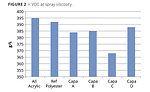
The low viscosity of polycaprolactones effectively reduces VOC in formulations that already can be considered to be high solids. It makes it appropriate to refer to them as reactive diluents. The additional reduction in Capa C further pronounces the effect but also highlights the importance to use a low-viscosity HDI-trimer to reach low-VOC values (Figure 2).
Abrasion Resistance
Improved abrasion resistance is a recurring property in different application areas of polycaprolactones such as polyurethane dispersions and urethane acrylates, where they have shown to have significant impact compared to conventional polyesters. The aliphatic, semicrystalline structure of the cured film results in a low Tg, but the film has high mechanical strength. A high crosslink density in combination with a low Tg results in a rubbery, elastomeric character of the film, which is beneficiary for good abrasion resistance. Results of the Taber abrasion test are shown in Figure 3.
Impact Resistance
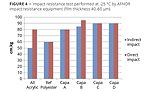
The aliphatic structures of polycaprolactones significantly improve impact resistance. In order to stress this, further tests were performed on metal panels that were heated at 80 °C for 24 h in order to ensure full cure, and then cooled down to -25 °C for 3 h in order to check mechanical properties at cold climates. Results show that the low Tg of caprolactones also decreases the Tg of cured coatings, a property which, for example, can improve stone chip resistance in automotive coatings and rain erosion resistance for windmill blades in harsh climate conditions (Figure 4).
Scratch Resistance
Scratch resistance was evaluated by a method where an indenter with a progressive increased load was applied on the coating. As long as no scratch was observed, only an elastic deformation of the surface was formed, which was quickly regenerated by reflow. When the load was further increased into the plastic deformation region, scratches were eventually regenerated as long as temperature was above Tg. Eventually the load increased until the point where an irreversible scratch was formed, and the coating was permanently fractured (which was monitored).
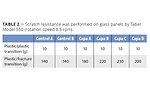
Results show the effect on Tg from addition of caprolactones, which reduce brittleness and risk of creating permanent scratches (Table 2). Semicrystallinity introduced by polycaprolactones also reduces internal stress and increases viscoelastic properties, which also improve reflow properties.
Flexibility
Polycaprolactones improve elongation and flexibility, especially compared to pure acrylic systems. The effect is pronounced by the increase of strain when the amount of polycaprolactones is further increased. Varying the amount and functionality of the caprolactones does not compromise the strength and toughness (Figure 5).
UV Resistance
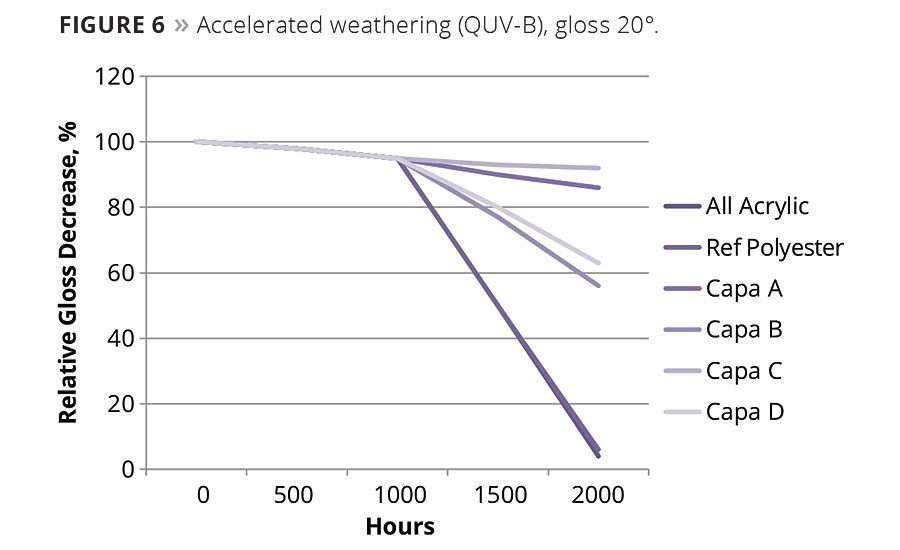
Clearcoats were formulated without additional UV absorbers and hindered amine light stabilizers, applied onto a white basecoat and exposed to QUV-B for 2000 h using a standard cycle. The aliphatic structure and the very low acid number (< 0.25 mg KOH/g) provide both good UV resistance as well as very good hydrolytic stability. The resistance is better than the pure acrylic system, and the effect is even more pronounced in the formulation with a double amount of caprolactones (Capa C) (Figure 6).
Waterborne Formulations
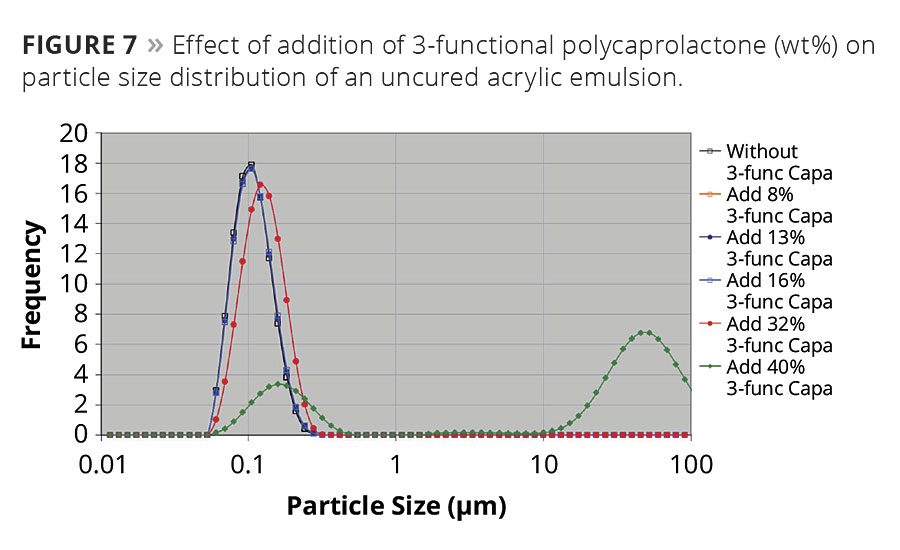
Polycaprolactones can conveniently be used in both solventborne and solvent-free formulations. They are, however, essentially hydrophobic, which makes them impossible to dilute and dissolve directly into water. And since they have an extremely low acid number it is not possible to make them water-dilutable by neutralizing carboxylic groups. They can, however, be used as resin modifiers in waterborne systems based on acrylic emulsions when they are allowed to migrate into the emulsion droplets without adding additional emulsifiers. Since both the hydrophobicity and viscosity of caprolactones are governed by molecular weight, it is preferred to use low-molecular-weight caprolactones with high hydroxyl content. Both properties make them easier to migrate into the emulsion droplets during a required dispersion process. They form a complex equilibrium in this kind of system, where molecular weight and viscosity of the acrylic, but more importantly type and amount of emulsifiers, have a crucial impact on the success. The amounts of different polycaprolactones that can be added to a certain acrylic emulsion vary and might not always be successful. It is case sensitive. The example in Figure 7 shows that in this particular commercial emulsion up to 16 wt% of the 3-functional polycaprolactones can be incorporated, which is shown by an intact particle size distribution. However, with the addition of 32 wt%, the peak is moved towards higher emulsion droplets, and with the addition of 40 wt% a distinct bimodal particle size distribution is formed, which indicates a fast phase separation.
General benefits of polycaprolactones in waterborne systems are similar to solventborne systems. Even VOC can be reduced since the need for fast-evaporating co-solvents is lessened. Influence on scratch resistance and impact resistance can be even more pronounced in these cases, since molecular weights of waterborne acrylics in many cases exceed a conventional solventborne acrylate. The influence of the addition of low-Tg and semicrystalline polycaprolactones can, in this case, be even more significant.
A well-known drawback with WB 2K PUR systems is that it is more difficult to achieve high gloss and low haze due to the topography of a cured surface with patterns formed by the remains of the emulsion droplets. This effect can be reduced by adding an organic co-solvent to reduce Tg and minimum film formation temperature, which promotes the coalescing process and levelling during physical drying. Due to the low viscosity and perfect compatibility with acrylic resins, polycaprolactones can fill the same function in a 2K WB formulation. Since caprolactones remain in film, in contrast to fast-evaporating organic solvent, physical drying and levelling can be prolonged, allowing even better levelling. It can be seen by an increase in gloss but is especially pronounced when haze is reduced (Figure 8).
In solventborne and solvent-free systems caprolactones can naturally be referred to as reactive diluents due to their low viscosity, but in waterborne emulsion-based systems they can more correctly be regarded as reactive coalescing agents.
Conclusions
Caprolactones are aliphatic, liquid, solvent-free polyols suitable as reactive diluents. They are prepared by ring-opening polymerization, which results in low acid numbers and exactly defined functionality that make them versatile tools when formulating various crosslinked coatings. When partly replacing conventional hydroxyl functional resins, they should be regarded as normal raw materials, but formulations should be balanced in order to take advantage of the unique properties of polycaprolactones. Even if they are aliphatic, semicrystalline polyols with low Tg, a balance can easily be found where flexibility, impact resistance and abrasion resistance can be significantly improved without compromising hardness or chemical resistance. This makes them suitable for harsh applications where good mechanical properties are needed in order to perform well in situations where properties like stone chip resistance, abrasion resistance and rain erosion resistance are of high importance.
Polycaprolactone polyols should preferably be used as resin modifiers in formulations that are needed to be taken to the next level. They can be used in formulations that need a twist in the right direction to meet VOC regulations and tough technical demands.
Results at a Glance
- Caprolactone polyols provide a versatile tool to fine-tune and optimize existing coating formulations.
- The aliphatic and semicrystalline nature of caprolactones can have a major impact on the mechanical properties of coatings.
- Their well-defined structure guarantees robust and reproducible final properties.
- The liquid and low-viscous nature of caprolactones makes them suitable as reactive diluents in solventborne formulations as well as reactive coalescing agents in waterborne formulations.
Acknowledgements
The author wishes to acknowledge the contributions of Aurore Jomier and Rolf Klucker at Vencorex.
References
1. Hill, L.W. Mechanical Properties of Coatings – Waterborne, High Solid & Powder Coatings Symposium March 2000.
2. Ma, Z. et al. Biodegradable Polyurethane Ureas with Variable Polyester or Polycarbonate Soft Segment: Effect of Crystallinity, Molecular Weight and Composition on Mechanical Properties – Biomacromolecules 2011, 12, 2365-3274.
3. Ardaud, P. et al. Waterborne and Solvent-Based Surface Resins and Their Applications, Vol III Polyurethanes, John Wiley & Sons, 1998.
4. Film Formation in Waterborne Polyurethanes: Surface and Bulk Morphology Development.
For more information, email par.jorgensen@perstorp.com.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!