The Science of Corrosion-Busting Smart Coatings














When it comes to fighting corrosion, industry needs coatings and materials that work smarter, not harder. The science of encapsulation has enabled the development of smart, self-healing materials that can stop corrosion when it starts. What is the science behind these materials, and what are the current limitations? Let’s take a closer look at where encapsulation technology is now and where it may take us in the future -for corrosion control and beyond.
The Corrosion Conundrum
Corrosion is a naturally occurring process that starts very small, with invisible changes to the physical structure of a material (usually a metal). These changes can be caused by a chemical reaction when metals are exposed to water, acids, gases or even chemicals produced by microbes (called microbially induced corrosion, or MIC).
But these small changes add up to big effects over time. Corrosion weakens the physical structure of the material and eventually leads to failure of the affected component. By the time corrosion is visible to the eye, the damage can be very costly to repair.
National Association of Corrosion Engineers (NACE) estimates that the direct costs for preventing, mitigating and repairing corrosion in the United States are $276 billion annually, and indirect costs such as lost productivity, litigation and environmental mitigation may push the total corrosion bill to more than $550 billion.
The many and varied methods used to protect infrastructure and equipment from corrosion include organic and metallic protective coatings; corrosion-resistant alloys, plastics and polymers; corrosion inhibitors; and cathodic protection. Each of these methods has a place, depending on the type of equipment or structure to be protected and the environment in which it resides.
Covering Up the Problem?
For many situations, brushing on a protective paint or coating is the simplest and most cost-effective mitigation strategy. There are many types of coatings available that offer some level of corrosion protection. At their simplest, protective coatings provide a barrier that prevents moisture and bacteria from reaching the metal surface. In theory, this will prevent development of conditions that lead to corrosion.
However, in practice, coatings will develop microflaws over time, which allow water, oxygen and bacteria to penetrate, providing a perfect environment for rust. Corrosion can start with microscopic flaws that are invisible to the human eye. Once started, corrosion can continue to spread underneath the coating, unobserved by casual visual inspection. By the time the problem becomes visible, considerable damage can be done.
More sophisticated methods may use a sacrificial element such as zinc, which is mixed right into the coating. These sacrificial elements slow down corrosion because they are more reactive than the metal they are protecting; that is, they quickly oxidize when exposed to oxygen and water, rendering these molecules harmless before they have a chance to react with the protected metal. Zinc-infused coatings provide longer-lasting protection than standard moisture barriers, but ultimately have the same problem.
The Rise of Smart, Self-Healing Coatings
Today’s most sophisticated corrosion-protective coatings go beyond moisture barriers and sacrificial elements. Advances in microencapsulation have enabled development of smart coatings that can heal themselves if damaged.
Smart coatings contain microcapsules or capsules that break open when exposed to a particular trigger. Microencapsulation allows an active ingredient, such as a healing agent, to stay enclosed and protected from the environment until it is needed (Figure 1). For example, a self-healing coating may release a chemical when the coating is damaged, automatically fixing microflaws that can allow corrosive agents to penetrate the coating (Figure 2).
Most existing self-healing materials contain a healing agent within capsules that break open when the coating is physically damaged. A catalyst is mixed into the coating or contained within its own microcapsule to act as a curing agent. When the healing agent is released and comes in contact with the catalyst, it creates a chemical reaction that hardens the healing agent, resealing the coating. All of this can happen at a microscopic level, long before flaws in the coating can be detected by the naked eye.
This self-healing quality can significantly increase the level of protection compared to traditional coatings. However, standard existing technologies have a few drawbacks:
- Most microcapsules are not functionalized to react to corrosion, meaning they only will break open when structural damage is caused to the coating. They are not able to detect and react to the presence of physicochemical markers of corrosion.
- The catalyst and healing agent must be mixed into the coating separately and stay physically separated until the healing agent is needed. Because the exact location of each molecule of healing agent and catalyst is randomized throughout the mixture, they may not be in close enough contact to react when the microcapsule does break open. This is especially true in dual-capsule mixtures, which require a healing agent-containing capsule and a catalyst-containing capsule to both break open at the same time in near proximity.
- Healing agents used in these coatings tend to be highly unstable, reacting readily with water and oxygen. This limits the shelf life of the product. If the healing agent reacts within the microcapsule before it is needed, it will no longer be able to provide self-healing properties when the need arises.
One-Step Corrosion Detection and Mitigation
Scientists at Battelle have been working to overcome these problems with the development of a single smart bead that can both detect and heal corrosion in one step. The Battelle Smart Corrosion Detector® bead is a microscopic bead that can detect corrosion forming on a metal substrate, deliver a payload to heal damage caused by the corrosion, and provide an early warning sign that corrosion is present.
The spherical capsules, 30 to 50 µm in diameter, resemble a fine, whitish powder in bulk. They are designed to be mixed into coatings that protect critical infrastructure from corrosion. They are different from standard smart bead technologies in two important ways:
- The coating of the microcapsule is functionalized, meaning it detects and reacts to microscopic signs of developing corrosion. This means that the coating does not have to be physically damaged to trigger the release of the healing agent; the presence of corrosion is itself the trigger.
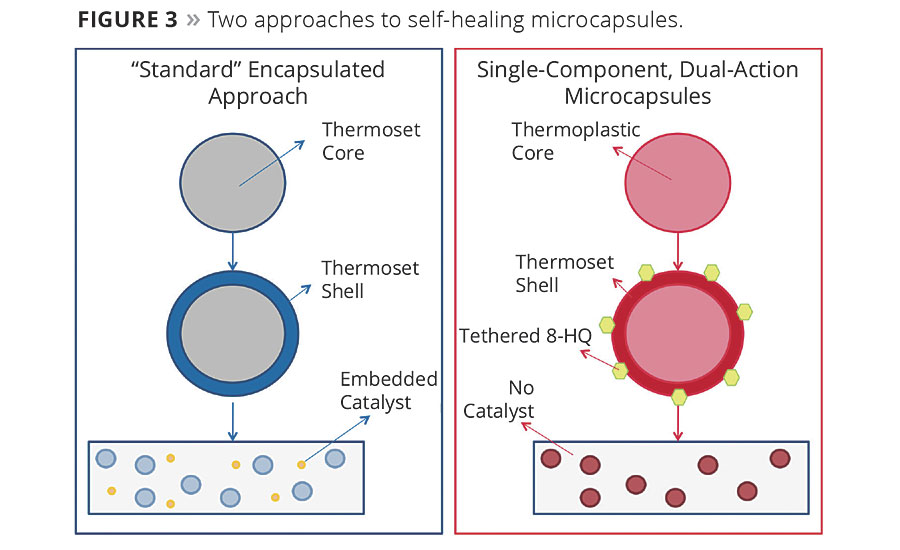
- It is a single-component system, which does not require the addition of a separate catalyst to cure the healing agent. The healing agent is instead cured by the corrosion byproducts (Figure 3).
In addition to releasing self-healing chemicals, the smart capsules detect and reveal corrosion forming on metal before it is visible to the naked eye. When corrosion is present, the capsules’ surfaces undergo a chemical reaction (Figure 4) that causes them to fluoresce (which can be detected with an ultraviolet [UV] light or Terahertz imaging). The fluorescence is a prompt indicator to maintainers that corrosion has initiated and provides them with the opportunity to mitigate the underlying problem early on, while the healing agent immediately repairs the corrosion damage and slows the corrosion process. The timely discovery and remediation of corrosion can result in significant time and cost savings as well as improved structural reliability.
Another key advantage is that moisture and oxygen permeability do not affect the stability of the healing agent. This means that the healing agent is very stable and will not cure inside the microcapsules, extending the usable life of the product.
The Science of Encapsulation
The Battelle Smart Corrosion Detector bead is just one application of sophisticated encapsulation methods under development at Battelle. The “Encapsulation By Design” initiative is focused on developing microcapsules that respond to specified triggers such as pressure, temperature, pH level, dilution, UV light or exposure to specific chemical or biological signals (Figure 5). Encapsulation technologies are transforming industries from agriculture to consumer products.
Encapsulation is the process of enclosing an active ingredient inside a protective shell, usually a polymer. Encapsulation allows reactive chemical components to be safely stored within a mixture until they are released by a specified trigger. This means that chemicals that react with each other, such as bleach and activators or healing agents and catalysts, can be safely combined in a single, shelf-stable product. Without encapsulation, the ingredients would have to be kept separate until the moment the user wanted them to be activated. Encapsulation also protects active ingredients from degradation and ensures that they will activate at the time and place where they are needed and not before, making it ideal for timed-release applications.
Materials can be encapsulated by several methods:
- Phase separation;
- Spray drying/spray congealing;
- Solvent evaporation;
- Coating.
The choice of particular method and shell material will depend on the physiochemical properties of the active substance and the desired particle size and release characteristics. Materials engineers can create microcapsules that respond to different triggers by altering the physical and chemical properties of the polymer shell. From a technological point of view, the successful selection of a preparation method will be determined by the ability to achieve high loadings with the active substance (high encapsulation efficiency), high product yields and the potential for easy scale-up.
The encapsulation technology developed by Battelle is based on a particle-forming polymerization approach. This method is amenable to encapsulation of both solid and liquid active ingredients. Particles can be created from nanometer to micron size. The process can be used to encapsulate a wide variety of active ingredients.
In this encapsulation process, the active ingredients are suspended in the medium using a stabilizer along with the desired shell-forming monomer and initiator (Figure 6). The initial polymerization occurs in solution. As the molecular weight increases, the polymer precipitates onto the active ingredients. The key to the success of dispersion polymerization is the choice of stabilizers and the types of monomer and solvents. If the active ingredients are water insoluble, either aqueous dispersion polymerization or other particle forming polymerization (such as emulsion and suspension polymerization) could be employed.
Beyond Corrosion: The Future of Smart Coatings
The applications for smart encapsulation technologies go far beyond corrosion detection and resistance. Similar technologies are already in use in a smart laundry capsule for one-step cold water bleaching, and may soon show up in additional consumer products and agricultural formulations. Eventually, they may be used for development of shelf-stable vaccines or delivery of biologic medicines.
There are additional applications within the coatings world as well. For example, a curing agent could be encapsulated within a shell that releases with exposure to UV light or another selected trigger. This would allow one-step application of coatings and resins that require a curing agent, without requiring on-site mixing.
Antimicrobial coatings are another potential growth opportunity for the coatings industry. Adding antimicrobial properties to coatings for industrial, or oil and gas equipment would offer another avenue of protection against microbially induced corrosion. Antimicrobial coatings could also be used in hospitals to reduce the spread of hospital-related infection, or in schools and other public buildings to slow the spread of colds and flus.
Encapsulation could be used to develop stimuli-responsive coatings and materials to inhibit targeted bacteria. These materials actively detect the presence of a microbe and generate a response such as the release of a biocidal agent. By calibrating the polymer coating to species-specific biomarkers, stimuli-responsive materials can be targeted to specific bacteria such as E. coli.Specific proteins on the bacterial cell wall could be used as a release mechanism to trigger the release of a biocide that neutralizes the pathogen.
As encapsulation technology evolves, the industry is sure to find additional applications. Targeted-release microcapsules will soon allow the formulation of new paints and coatings that are longer lasting, more protective, and easier to store and apply. Many of these applications are potentially market disrupting. Companies who have the creativity and vision to imagine new uses for encapsulation will have a powerful new competitive advantage in the marketplace.
Suggested Reading
1. Benkoski, J.J.; Srinivasan, R.; Maranchi, J.P. Self-healing coatings consisting of a polymeric microcapsule enclosed with a metallic shell. US20110293958A1 (2011).
2. Braun, P.V.; Cho, S.H.; White, S.R. Self-healing coatings comprising siloxane polymerizer and activator. WO2007082153A2 (2007).
3. Chung, K.; Lee, S.; Park, M.; Yoo, P.; Hong, Y. Preparation and characterization of microcapsule-containing self-healing asphalt. J. Ind. Eng. Chem. (Amsterdam, Neth.) 29, 330-337, doi:10.1016/j.jiec.2015.04.011 (2015).
4. Samadzadeh, M.; Boura, S.H.; Peikari, M.; Kasiriha, S.M.; Ashrafi, A. A review on self-healing coatings based on micro/nanocapsules. Prog. Org. Coat. 68, 159-164, doi:10.1016/j.porgcoat.2010.01.006 (2010).
5. Shokuhi-Rad, A.; Ardjmand, M. Various microencapsulation techniques to gain self-healing polymers. Sci. Int. (Lahore, Pak.) 21, 109-121 (2009).
6. White, S.R. et al. Autonomic healing of polymer composites. Nature(London, U. K.) 409, 794-797, doi:10.1038/35057232 (2001).
7. Yang, J.; Huang, M. Microencapsulation of organic silanes and use in self-healing coating materials. WO2013137828A1 (2013).
8. Zhao, Y.; Zhang, W.; Liao, L.; Li, W.; Xin, Y. Microencapsulation of epoxy resins for self-healing material. Adv. Mater. Res. (Zuerich, Switz.) 148-149, 1031-1035, doi:10.4028/www.scientific.net/AMR.148-149.1031 (2011).
For more information, e-mail lalgudir@battelle.org.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!