Traditionally, organo modification of silicones has involved incorporating polyether, alkyl and phenyl groups onto the silicone backbone. For this study, carbinol functionality has been incorporated onto the silicone backbone using a new capability in Dow Corning to functionalize silicone resins/polymers with organic moieties etc. These novel materials will be capable of co-reacting with many organic cure chemistries, resulting in improved compatibility and other performance aspects of the final coating.
This paper describes the initial study of carbinol functional materials where prototype materials were cold blended into standard parquet lacquer formulations. The next stage of the program will investigate chemically reacting the functional groups into the resin. A range of carbinol fluid structures were evaluated in terms of their performance in UV and waterborne parquet-type formulations. The silicone chemistry is described, as well as the relationship between chemical structure and suitability for particular parquet lacquer formulations.

Silicone Additive Benefits for Parquet Coatings
For many coatings systems, the regulatory drive away from solventborne towards waterborne formulations has created performance issues. Coating formulators are less able to rely on the flow and leveling benefits provided by solvents. In addition, waterborne polymers may not provide the required level of gloss, foam control, blocking and mar resistance. New formulations include non-VOC-contributing additives to provide the needed performance. Silicone additives are well suited for use in parquet coatings to protect the coating surface from mechanical damage during processing, transport and use. They can also improve the visual appearance by helping to produce a smooth coating surface free of defects.
Current Silicone Technology
Traditionally, silicone polyether technology has been used to impart slip, wetting, leveling and defoaming. These copolymers will have typical surfactant features, i.e. hydrophobic and hydrophilic segments. The ratio of the hydrophobic/hydrophilic segments is important to achieve the required compatibility balance. A high hydrophobic content may lead to de-wetting defects e.g. fish eyes, craters. On the other hand, a high hydrophilic content can increase the solubility of the copolymer so that there is no driving force for accumulation at the coating/air interface during the drying process.
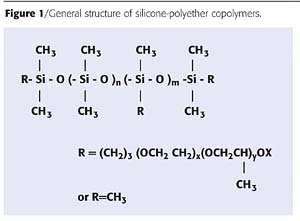
Figure 1 shows the general structure of a silicone-polyether copolymer. The polyether chains can be attached at points along the length of the silicone backbone, giving a comb-like structure. They can also be attached to the ends of the silicone polymer, giving telechelic, linear structures. The architecture of the copolymer has a profound effect on the behaviour as an additive. Optimized structures have been identified by designed experimentation to give suitable combinations of compatibility and desired effect, such as slip, leveling or wetting and defoaming. Compatibility is particularly important in clear parquet coatings, where gloss reduction or haze cannot be tolerated.
This type of silicone technology is already widely known. This paper will attempt to build on the current understanding by evaluating three alternative polymer structures with a carbinol functional group attached to the silicone backbone:
- ABA linear silicone carbinol fluid with (EO)1OH functionality;
- Silicone carbinol fluids with novel hydrophilic groups;
- Carbinol functional silicone resins.

ABA Linear Silicone Carbinol Fluid with (EO)1OH Functionality
Performance in UV Solventless Parquet Coating FormulationThe ABA linear samples tested varied in the length of the siloxane unit. Though these materials are soluble in polar solvents, they are extremely hydrophobic and, therefore, not water soluble or dispersible. Tests were performed in a UV system only. The evaluations of ABA linear carbinol functional fluids were performed using the formulation shown in Table 1.
The performances of these various molecules in these formulations were studied, and the following properties were evaluated: mar resistance; gloss/haze; slip/friction; and surface appearance (leveling, compatibility).
Mar resistance is an important property for parquet. The three materials all have the effect of improving the mar resistance compared to no silicone addition, as shown in Figure 2. The mar resistance also increases as the number of siloxane units in the siloxane backbone increases in number from left to right (1 = excellent appearance, 5 = poor appearance).
Another important property is the compatibility in terms of no cratering or leveling irregularities. The silicone fluids should have no negative effects on the appearance of the final coating. The results in Figure 3 demonstrate an improvement in the appearance as the number of siloxane units in the siloxane backbone increases in number from left to right (1 = excellent appearance, 5 = poor appearance).
The slip performance or coefficient of friction was also tested and compared to the control. This also gives a measure of whether the silicone tested comes to the air-liquid interface. As can be seen in Figure 4, the slip is reduced, showing their presence at the surface for all three molecules tested.

The advantages of moving to waterborne systems in terms of healthier, safer and environmentally more acceptable coatings are obvious. However there is also one significant disadvantage. Waterborne coatings usually contain surfactants (e.g. binder, dispersant, wetting agent etc), which have the negative effect of stabilizing foam from air incorporation during the production and application of the coating. However, it is important for these waterborne coatings to contain wetting agents due to the high surface tension of water, which can cause surface defects such as craters and poor wetting on substrates. Dow Corning promotes various silicone polyether technologies to be used as wetting agents. One successful product in particular, a trisiloxane polyether (Figure 5), is sold as an anti-crater additive and acts by lowering the surface tension of the liquid coating and thus improves substrate wetting.


A range of silicone carbinol fluids with novel hydrophilic groups were synthesized for this evaluation with the hope that good wetting and good compatibility could be achieved without compromising the foaming behaviour of the liquid coatings.
Five different structures were designed and performance tested. These structures contained the same siloxane chain length but varied in the nature of the carbinol functional hydrophilic group. (The exact nature of these structures is proprietary.)
The evaluations of these fluids were performed using the formulations shown in Tables 2 and 3.

- 1. Mar resistance
2. Gloss/Haze
3. Slip/Friction
4. Surface appearance (leveling, compatibility)
5. Foam stability
6. Blocking
When mixed at high shear, these materials did not show foam stabilization typically seen with wetting surfactants (Figure 8).
The addition of the silicone-carbinol fluids to the lacquers did not adversely affect the slip, blocking or gloss of the final coating. No change in mar resistance was observed.
The effect on wetting was measured by surface tension (Figure 9). With the new silicone-carbinol fluids evaluated, only a small change in surface tension was observed.
At this time, the trisiloxane material, with three Si atoms and pendant EO groups, still gives the best results for wetting. The new hydrophilic silicone-carbinol fluids do not give the problem of foam stabilization as seen with the trisiloxane material, but the degree of polymerization of the silicone backbone and/or the molecular weight of the hydrophilic group is clearly still too high to achieve the excellent mobility in solution and very efficient packing at the interface achieved by the trisiloxane material.

The next stage in the development of these materials will be to look at lowering the degree of polymerization of the silicone backbone and the molecular weight and structure of the hydrophilic group to achieve excellent wetting without foam stabilization.
Finally, the next class of carbinol functional materials, carbinol functional methyl resins, was evaluated in formulations included previously. A comparison was also made to non-functional methyl resins. Materials are not soluble in water and therefore were delivered into the coating in emulsion form.

Earlier we saw that linear silicone-carbinol fluids gave no benefit in mar resistance in waterborne coatings. The data in Figure 12 shows the positive benefit of adding a more rigid, three-dimensional structure to the coating to improve mar resistance. The substitution of phenyl groups into the carbinol resin also improves the mar resistance at lower addition levels compared to the non-functional methyl only resins.
Of course it is important to ensure that other properties required in the coatings remain unaffected by the addition of the silicone resins e.g., gloss, slip. Figures 13 and 14 illustrate that in the UV and air-drying formulations tested, the phenyl substituted carbinol resins had no negative impact on slip or gloss.
Conclusions
Silicone additives have progressed enormously since the first use of PDMS fluids in solventborne coatings. New chemical structures and delivery forms promise even greater improvements. This paper summarizes the performance of different silicone technologies in various parquet coating formulations.Short chain ABA linear silicone-carbinol fluids with (EO)1OH functionality have shown good compatibility in UV solventless coatings. They can migrate to the coating surface, giving increased mar resistance and slip. Their hydrophobicity does not allow them to be used directly in waterborne coatings. Further work is planned using an emulsion delivery system to determine their effects in waterborne coatings.
Increasing the hydrophilic character of the silicone-carbinol fluids allows them to be directly incorporated in waterborne systems. Their compatibility in waterborne coatings is dependent on the balance between the length of the siloxane backbone and the molecular weight of the hydrophilic group. They do not cause foam stabilization seen with other surfactants, but more work is needed on optimizing the structures of these materials if benefits in wetting and mar resistance are to be achieved.
Moving from a linear fluid to a rigid, three-dimensional resin brings improvements in mar resistance without adversely affecting the slip or gloss level of the coating. Carbinol functional resins show improved mar resistance compared to non-functional resins, but this must be balanced with phenyl functionality to ensure compatibility in the coating. Further work is planned to determine improvements in performance by reacting carbinol resins with organic binders.
Acknowledgements
Donna Perry and Vicky James are technical service specialists for Dow Corning Europe. Jerry Witucki is a technical service specialist with Dow Corning Corporation. The authors wish to thank Frances Fournier, Gary Wieber and Mike Ferritto at Dow Corning Corporation and Katrin Langosch at Worlee Chemie GmbH for their valuable contribution to this work. They would also like to thank UCB, Alberdingk Boley, BASF and Rohm and Haas for their contributions to test formulations.References
1. Owen, MJ, "The Surface Activity of Silicones: A short review", I&EC Prod. Res. Dev., 10 (1980), 97.2. W.H. Pushaw III in Handbook of Coatings Additives (L.J. Calbo, ed.), Marcel Dekker, Inc., New York, 1987, p.271.
For further information contact, Donna.perry@dowcorning.com, Vicky.james@dowcorning.com, or Gerald.Witucki@dowcorning.com.
This paper was presented at the European Coatings Conference "Parquet Coatings III", which took place November 2004, in Berlin. Information is available at Vincentz Network, amanda.beyer@coatings.de.





