GREENKOTE: Corrosion-Resistant Poly-Metal Diffusion Coatings

The corrosion protection requirements imposed on painted parts and components by industry have become increasingly more rigorous than they were even 10 years ago. Anticorrosive coating and painting manufacturers have attempted to meet the more stringent coating corrosion-resistance requirements by the addition of numerous components aimed at increasing the corrosion resistance of their coating compositions. A solution that addresses these stricter demands is to combine base metal sacrificial metallic coating with paints. With a sacrificial coating, the base coating supports the paint layer in damaged or weak areas, thereby providing a more comprehensive and longer-lasting corrosion protection system.
To address the more stringent corrosion requirements, a number of coating processes have been developed. These include electroplating, hot-dip, galvanizing and sherardizing. Though these newer coating processes improve corrosion resistance, they still incurr the following problems: poor adhesion of the paint to the coating and destruction of the paint film after white corrosion begins to form. The first problem occurs with electroplating and hot-dip coatings, while the second occurs for all types of coatings, including mechanical galvanizing and sherardizing.
Electroplating
In the electroplating process, coatings composed of Zn-Fe, Zn-Ni, Zn-Co, Zn-Cd alloys were developed and produced. However, the added complexity and sensitivity of the coating precipitation process and the resulting need for more precise production control led to a substantial increase in coating production costs. Also, the addition of potential environmentally dangerous elements in the coatings, such as Ni, Co and Cd, led to higher waste treatment expenditures.
Hot-Dip
Another commonly accepted method for applying a corrosion-resistant zinc-based coating is the hot-dip (or galvanizing) process, in which treated parts are immersed in a molten zinc bath. This method is widely used for applying coatings onto large-sized products, and also for a continuous process of applying protective coatings on metal sheet and wire.
In the 1970s, hot-dip processes were modified to use a bath containing aluminum, as well as zinc. Coatings produced using these hot-dip processes have various commercial names; however their chemical composition is essentially the same. The coating, known in Europe and the United States as Galfan, contains 4.7 - 5.2% by weight of aluminum. The Galvalum coating, commercially produced in the United States, contains approximately 55% by weight of aluminum.
Galfan coating is characterized by a 3- to 5-fold lower corrosive mass-loss rate than that of conventional hot-dip technology coatings, and also, by an increased period of time elapsed before the appearance of base metal red corrosion.
Although the introduction of relatively small amounts of aluminum (usually 4-7% by weight) is known to lead to an increased corrosion resistance of zinc coating, the electrochemical mechanism that leads to this effect is not apparent. The higher resistance of Zn-Fe, Zn-Ni, Zn-Cd alloys in comparison with pure zinc can be attributed to their higher electrode potential, as the electrochemical potential of the second component of the alloy is higher than that of zinc, as shown in Table 1.
Conversely, in zinc alloys with aluminum and magnesium having electrochemical potentials equal to -1.663 V and -2.37 V, respectively, the situation is reverse. From the electrochemical point of view, zinc alloys with aluminum and/or magnesium should be less corrosion resistant.
The increase in the corrosion resistance of zinc-aluminum coatings produced by hot-dip technology has been achieved by making the chemical composition of the coating more complex. Adding magnesium and silicon to the coating composition made it possible to bring the corrosion resistance of such coatings up to the level of today's industrial anticorrosive coating requirements.
According to J. Tanaka et al.,1 a Zn-Al coating with Mg and Si admixtures has a complicated phase composition caused by phase transformation processes at the transition from bath temperature to room temperature. In addition to a Zn-Fe intermetallide layer adjacent to the base metal, phases of almost pure zinc, almost pure aluminum and eutectic Zn-Al compositions, with approximately 5 - 6% by weight of Al, are also observed. According to these authors, almost pure aluminum does not corrode due to its self-passivation capability.
The increased corrosion stability of Galfan-type coatings seems to be due to the fact that the eutectic zinc-aluminum, when subjected to corrosion, forms insoluble compounds, which fill coating defects such as pores and cracks. In essence, the activity of these insoluble compounds provides an effect of coating self-passivation similar to that observed in pure aluminum and its alloys.
However, coatings applied using this technology on parts that have a complex shape fail to provide a uniform thickness. Another common drawback of zinc-based coatings applied by both the electroplating and hot-dip processes is their low adhesion to secondary coatings. White corrosion of zinc, developed in the vicinity of pores and defects in the paint, leads to the mechanical destruction of the second coating. As the corrosion process advances, its products displace the paint, which exhibits blistering.
Sherardizing
Sherardizing is a process where parts are heated for several hours in a closed, usually rotating, container together with zinc powder at temperatures of 370-450 ºC. As a result of this process, two intermetallide Zn-Fe phases are formed on the substrate surface. The first phase is usually only several microns thick. It is adjacent to the base metal and contains approximately 20% by weight of iron. The second phase, which forms the main part of the coating thickness, contains less iron, usually up to 12% by weight of iron.The process temperature approaches the zinc melting temperature (~ 419 ºC) and sometimes exceeds it. To prevent the powder from fusing and/or sticking to the substrates, the zinc powder is diluted with inert filler, such as sand, aluminum oxide and so forth.
As a result of the Sherardizing process, a relatively rough and porous coating is formed, which adheres uniformly to the profile of the substrate. The coating also serves as a good substrate upon which to apply a second coating or paint with good adhesion.
But white corrosion of zinc, developed in the vicinity of pores and defects of the paint, leads to the mechanical destruction of the paint. As the corrosion process advances, it displaces the paint, which exhibits blistering.
GREENKOTE™
The new sacrificial coating group, known as GREENKOTE PM-group, provides excellent paint adhesion and a low white corrosion rate, which prevents the destruction of paint while improving corrosion resistance.The corrosion protection system based on these sacrificial coatings was tested and improved by Volkswagen and GM for heavy-duty corrosion areas, and by Motorola as a cheaper and better alternative for powder paints in outdoor applications.
Accordingly, it is the principal objective of the GREENKOTE PM-1 coating invention to overcome the above-mentioned disadvantages and provide a diffusion coating that possesses all of the advantages of the Sherardizing coating process, but attains a high corrosion resistance rate as well as a low white corrosion creation rate. More specifically, the objective is to provide a polymetallic Zn-Fe-Al diffusion coating for iron-based item surfaces. A further object of the GREENKOTE PM-1 coating is to provide the composition of the saturating powder mixture that is required to implement the diffusion coating process to produce the coating.
The polymetallic GREENKOTE PM-1 coating has a multiphase structure. The first two layers adjacent to the metal substrate contain Zn-Fe intermetallics, as is common for regular Sherardizing. The total thickness of these layers can equal or even exceed 100 microns. The optimal thickness for each particular instance is selected depending on the requirements for that instance.
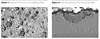
In contrast to Sherardizing, the main Zn-Fe intermetallic diffusion layer contains Zn-Al-Fe inclusions (Figures 1 and 2). The composition of the phases of these inclusions depends to a great extent on the cooling rate and the initial saturation powder composition. The components Zn, Al and Fe must always be present in these phases. Other metals may also be included.
The coating is obtained by a diffusion process that is realized by heating the products at temperatures of 370 - 450 ºC in a saturating powder mixture environment in a closed container, wherein the coating composition comprises aluminum, as well as iron and zinc, as alloys and intermetallics. In the preferred embodiment, particles of those alloys and intermetallics are distributed as Al-rich inclusions, serving as sacrificial phases, mainly on the surface of the coating.

Figure Descriptions
For a better understanding of the invention, reference is made to the accompanying Figures, in which Figure 1 shows Al-Fe-Zn inclusions on the coating surface. Figure 2 shows a cross-sectional view of an Al-Fe-Zn inclusion. The inclusions, having different structure than the main coating structure, are clearly shown. The average size of these inclusions is 46 microns. According to microanalysis data, the composition of these inclusions is as follows: 30% by weight of zinc, 39% by weight of iron and 30% by weight of aluminum. According to the Fe-Al binary phase diagram, the included particles are comprised of Fe-Al intermetallic and Zn-Al alloy. Apparently, the presence of patches, having such a composition, results in a higher coating corrosion.Figure 3 presents salt spray test results for different coating compositions and processes. It shows mass losses of various coatings at salt spray test and provides an illustration of the high corrosion resistance of the proposed coating. The very low coating mass loss rate for the GREENKOTE PM-1 coating shows a very low Zn corrosion (white corrosion) rate - an additional advantage for paint base coating to prevent the destruction of the paint layer.
Figure 4 presents microanalysis data for coating and inclusion composition.

Preferred Embodiment Description
As described previously, the primary cause of corrosion in Sherardizing process coating products is the electrochemical reaction between the substrate (consisting mainly of iron) and the intermetallic Zn-Fe coating.To minimize the corrosion-producing effects of the electrochemical reaction between the substrate and the intermetallic Zn-Fe coating, the GREENKOTE PM-1 coating introduces sacrificial phases. These sacrificial phases protect Zn-Fe intermetallics from a direct electrochemical reaction with the substrate material. Inclusions formed from Al-Fe intermetallics and Zn-Al alloys can serve as such sacrificial phases. Al-Fe intermetallics and Zn-Al alloys, being more chemically active than intermetallic Fe-Zn, are not as likely to experience self-passivation as is aluminum. In Zn-Al alloys and Al-Fe intermetallics, a continuous passivation film of aluminum hydroxides is not formed.
In addition, products of aluminum corrosion possess low solubility in water. They precipitate on coating defects (cracks, pores, etc.), fill them and thereby considerably slow down the corrosion rate. This effect of aluminum corrosion product filling does not disappear, even after the sacrificed phase is consumed. Moreover, zinc corrosion products enhance this effect by precipitating on coating defects, and thus imparting the coating with higher corrosion resistance under salt spray conditions. This mechanism explains the higher resistance of the coating as compared with the well-known Galfan type Zn-Al coating (Figure 3).
During the coating process, zinc diffuses from the saturating powder environment and forms the layer of Zn-Fe intermetallics on the surface of the substrates. The powder mixture becomes enriched with aluminum. The reaction of aluminum with iron begins and Fe-Al intermetallics are formatted. This reaction occurs due to diffusion of iron from the Zn-Fe intermetallic layer into the Zn-Al pore powder grains. These inclusions are randomly distributed, for the most part on the Zn-Fe intermetallic surface, as Al-Fe intermetallics (Figure 1).
Conclusion
Today's coated metals must withstand harsher marine, industrial and rural conditions and more intensive usage than the metal-coated components had to as little as 10 years ago. Furthermore, the rising cost of raw materials, especially stainless steel, has caused manufacturers to turn to less-expensive materials that have poorer corrosion resistance and therefore require a better-performing coating. These industry conditions have created a demand for greater corrosion protection for manufacturers of metal components and their customers. As detailed in this article, GREENKOTE's sacrificial coating provides a solution that improves corrosion resistance.This coating process, as described, is taken from patent Pub. No. US 2004/0105998 A1 published on June 3, 2004.
For further information in the United States, contact Steve Broderick at 317/595.9535, or e-mail srbroderick@juno.com.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!



