Core Shell Nanoparticle Metal Oxide Infusion
















Acrylic resins have been manufactured since the 1940s, and today’s advanced techniques yield high-performance acrylics. Manufacturing processes also allow for the “customization” of acrylics. One method for customization uses multiphase or core shell polymerization (Figure 1).
Core shell polymerization has been utilized for many decades. When run effectively, the process can yield a product that is essentially two resins in one particle. The dual particle nature can be modified with differing species/hybrids in the core or shell. Many resin systems can be made in the nanoparticle range of 20-110 nanometers.
There are many benefits to having a smaller-particle-size acrylic emulsion. Lower-particle-size emulsions can be a benefit in wood or concrete coatings where penetration into the substrate is needed. The lower particle size allows for the further penetration into a substrate and also yields topcoats with higher clarity and better depth of image.
Another area that is very active in coatings research as well as material science is nanoparticle metal oxides, which can range from 10-100 nanometers. Examples of these metal oxides are aluminum oxide or silicon dioxide used for scratch and abrasion resistance. Materials such as zinc oxide, titanium dioxide or cerium oxide have been shown to offer enhanced UV resistance.
The current recommended route is the post addition of the nanoparticle metal oxide solutions to the coating, thereby making incorporation relatively easy. Incorporation of these materials can range from 1-3% on total formula weight for materials such as aluminum or silicone oxide for scratch resistance, up to as high as 5% for zinc or cerium oxide for UV protection. These materials migrate around the coating’s solvent medium in the water or solvent phase. They offer the ability to get between resin particles and intermix with other additives in the coating system.
One of the potential problems identified with nanoparticle systems is the stabilization in finished paints or polymers as a post addition. Over time, there is competition between the surfactant system in the latex particle and the stabilization of the nanoparticle dispersion. Another issue is agglomeration with the acrylic resin itself. We have found that these materials tend to agglomerate and grow in particle size, thus losing their nanoparticle size (Figure 2).
To prevent the flocculation of the nanoparticle dispersion, the particles need to be further stabilized. We have been working on a novel process that will incorporate the nanoparticle dispersion into our polymerization process. We have discovered a technique to utilize one of our poly-merization processes. Our multiphase core shell process allows us the flexibility to use single-stage monomer feeds or work with multiple, parallel monomer feed streams.
Experimental
We processed one of our acrylic polymers, (RayCryl 1096) with a 1% (RayPlus 1097) and 3.5% loading of a 10-nanometer aqueous solution of aluminum oxide to determine the effect on particle size and coating performance. We compared these to the standard acrylic and to a 1% post addition of the same solution.
The acrylic utilized was a 21 °C MFFT material with a 70-nanometer D50 particle size. The acrylic follows our core shell or multiphase process. Currently, it is utilized as a clear for decorative wood products and provides high gloss and good depth of image.
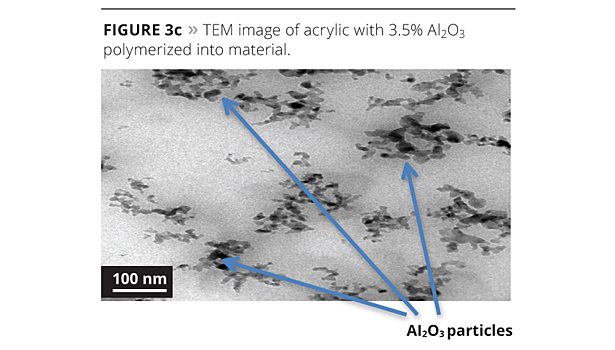
To determine the effect on reaction of the aluminum oxide solution in the polymerization, we examined each process (Figures 3a-3d) using transmission electron microscopy (TEM).
We also evaluated the acrylic versus the acrylic with two different levels of the nanoparticle aluminum oxide polymerized into the material as well as the post addition solution. Both particle size and minimum film formation temperature were measured. Results can be seen in Table 1.
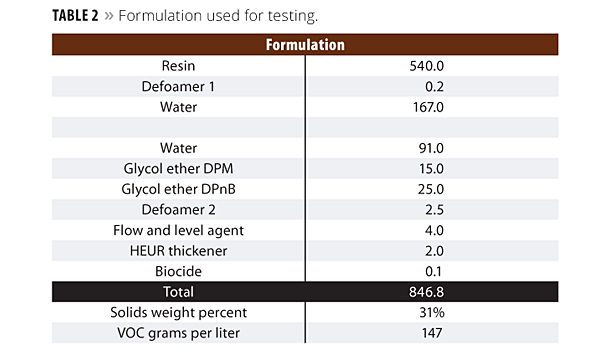
Chemical resistance was evaluated using over 24 reagents in a 1-hour spot test on maple veneer plywood. We evaluated Taber abrasion following ASTM D4060 using CS10 wheels with 1000 grams and 500 cycles. Hoffman scratch resistance was run on oak boards. All physical testing was conducted using the formulation in Table 2.
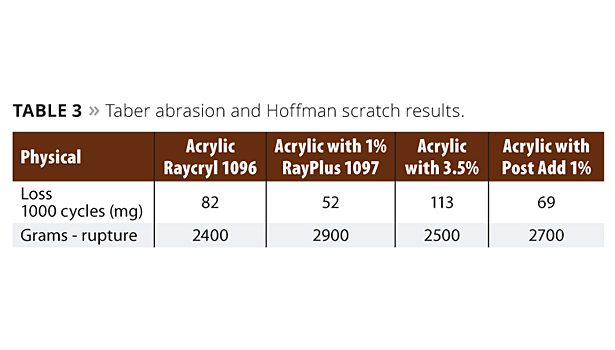
The chemical evaluation was conducted on maple veneer panels. Three coats were spray applied and cured for seven days. To run Taber abrasion, we applied three coats to the metal panels and cured for seven days. To evaluate Hoffman scratch, three coats were applied to red oak ¾-inch boards and cured for seven days. Failure analysis was conducted with a red dye solution, which stained the wood at the point of film destruction. Taber abrasion and Hoffman scratch results are shown in Table 3.
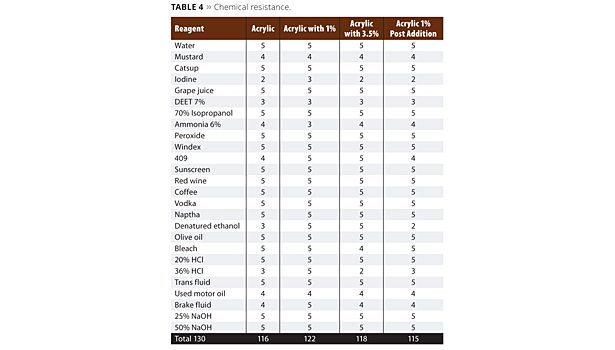
Table 4 shows the list of reagents used in a 1-hour spot test exposure. The grading scale was 1-5, with 1 being destruction of the film, and 5 having no effect on it. The higher the score, the better the performance of the coating. Evaluation was determined immediately after the reagent was removed.
Results
We wanted to determine the effect of varying the level of aluminum oxide in our process and also to determine the effect of post addition. We utilized several methods for evaluation in order to first determine what we were doing to the polymer system, and then to determine the physical performance characteristics each variable yielded.
When we ran transmission electron microscopy on the samples, we could isolate the aluminum oxide in each system that contained it. The base resin was used as a blank in order to compare our images and particle size analysis. When looking at the images, the 1% polymerized version has very good spacing of the nanoparticles in the system. When the level is pushed to 3.5%, there is good spacing, but there is also the start of some clustering of particles. This may have caused the coating’s performance to diminish somewhat. The post addition of 1% aluminum oxide to the base acrylic caused the formation of pockets of localized aluminum oxide that are not spaced as uniformly throughout the acrylic matrix. While the system still has performance advantages, it will eventually suffer the agglomeration as illustrated in Figure 2. The particle size analysis supports the mono-dispersed particles in the 1% polymerized versus the post addition.
The next step in our evaluation was to look at the film formation and particle size distribution characteristics. The base polymer, RayCryl 1096, had a minimum film formation temperature of 21 °C and a relatively mono-disperse nature at D10, D50 and D90. The particle size distribution for the 1% polymerized version maintained a pretty narrow particle size throughout the analysis range. When we increased the polymerized version to 3.5%, the particle size and distribution changed by the largest amount. The 1% post addition was similar in particle size to both the base acrylic and the 1% polymerized versions, however the volume and number average of the particles was larger than both the base acrylic and 1% polymerized versions. This indicates possible coagulation and reaction with acid groups or competition with the surfactant in the latex system.
Another interesting point is that in both cases of the polymerized versions the film formation temperature was increased only a couple of degrees. This however was not the case for the post addition of the aluminum oxide. The film formation temperature had only a slight increase over the base acrylic when aluminum oxide was introduced via polymerization.
In the physical evaluation of the resin systems we utilized the previous formulation for each product and tested it on various substrates. For the Taber abrasion test the performance improved at both the 1% polymerized and post addition levels. This was compared to the base resin. The polymerized version yielded a 36% improvement over the base acrylic, while the post addition produced a 16% improvement in abrasion resistance. The 3.5% polymerized version provided a 33% decrease in abrasion.
The Hoffman scratch test showed similar results. The 1% polymerized product offered a 21% improvement in scratch resistance; similarly the 1% post addition yielded a 12.5% improvement over the standard. Again, the 3.5% polymerized version fell short of the 1% additions to the system, increasing scratch by only 4%.
The chemical resistance data in Table 4 is very close in performance. Our base resin started with very good chemical resistance. The performance gain in this data set was improved denatured alcohol resistance as well as improved 409 cleaner resistance. Some notably difficult chemicals are Off bug repellent with DEET, iodine, ammonia and mustard. The mustard and iodine caused surface staining and will eventually recover and lighten. The ammonia penetrated the coating but also lightened. The DEET was very aggressive to the coating surface and softened it.
Conclusion
The use of nanoparticle oxide material does enhance performance of a number of resin systems. Nanoparticle aluminum oxide is being used to enhance performance for many applications. We saw the need to utilize the nanomaterial to further enhance our resin performance and developed a unique polymerization technique to functionalize the metal oxide and then trap the nanoparticle in our resin system.
In our evaluation we saw improvements in abrasion, scratch and chemical resistance over our standard RayCryl 1096. Utilizing our special technique we have determined that the loading level of RayPlus 1097 at 1% is the optimum level for best performance attributes. The aluminum oxide gets trapped and spaced uniformly throughout the resin matrix. While the 3.5% level does get trapped and spaced, it is too much for the resin system and opens the film up for degradation. The 1% post addition does offer performance improvement over the RayCryl 1096, but will suffer agglomeration over time. We will continue looking at other nanoparticle oxides in similar chemistry resin systems to further enhance our line of acrylic resin systems.
For more information, contact jschierlmann@specpoly.com.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!















