Silicone’s Positive Influences on Exterior Coatings

















The increase in home remodeling and improvements has spurred the growth of architectural coatings. Consumers see opportunities to remodel a home, and paint is a component that easily makes a home feel like new again.1 Consumers rely on exterior coatings to have excellent weather resistance, long-term durability and UV protection that can last 20+ years on average. There is also a desire for coatings to have a “clean” appearance and a decrease in the amount of cleaning needed to maintain this appearance over the years.
Self-cleaning and dirt-resistant coatings have become hot topics. Dirt repellency has always been important, but in the current climate it is now extremely crucial. Paint suppliers are hungry for marketing data, exterior testing, proof of concept on how to increase the dirt repellency of coatings, and to sell their paint as the best dirt-repellant technology. Silicones provide water-resistant properties that reduce the moisture content at the surface, thus increasing dirt repellency. This article will discuss the chemistry of silicones, multiple silicone technologies used in various paint formulations and the results of outdoor exposure testing with natural dirt collection.
Silicone Chemistry
The term “silicone” refers to compounds that have the empirical formula R2SiO, which is analogous to organic compounds with the formula R2CO or ketones.2 Silicon is similar to carbon in that it is tetravalent; however in its ground state there is no evidence that supports the formation of a double bond. Therefore, the true empirical formula should be written as R2SiO2(½) with four single bonds to silicon.2
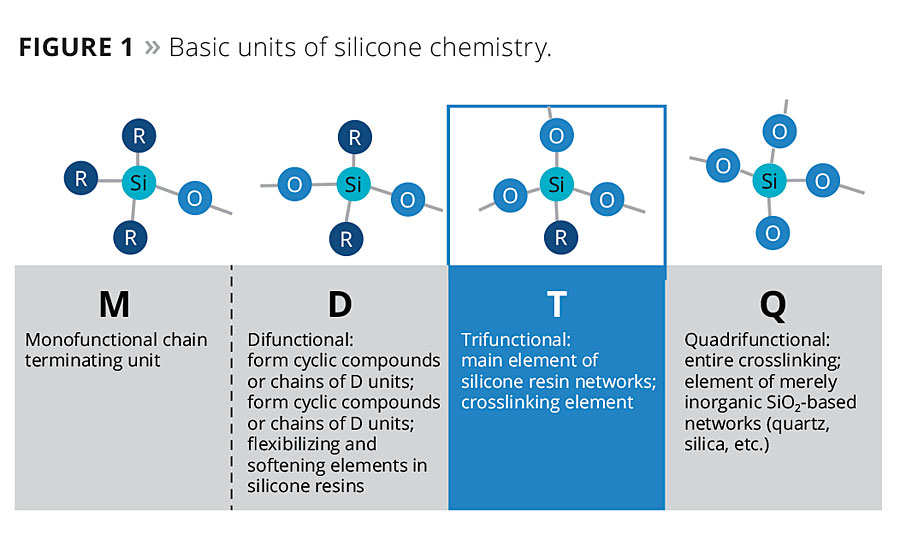
Silane is a term given to compounds composed of a single silicon atom. The amount of varying functional sites, Si-O, then creates a sub-classification as M, D, T or Q units, when describing the different silane chemistries. Figure 1 illustrates the nomenclature of silanes by referring the single Si-O bond as monofunctional or “M” units. The silane containing two Si-O bonds are referred to as difunctional or “D” units, and the trifunctional silanes are referred to as “T” units. The quadrifunctional silanes are referred to as “Q” units or known as the tetrafunctional silane. A material containing a silicon-oxygen (siloxy-) bond is termed siloxane. A siloxane that contains two silicon atoms bonded to each other through an oxygen bond is referred as a disiloxane, and a siloxane with three silicon atoms is a trisiloxane. A polysiloxane is a siloxane containing greater than three silicon atoms.2
Silicone Resin Emulsions, and Silicone Additive Chemistries and Behaviors
Numerous chemistries are reaching their centennial celebrations, which reminds us of the key advantages they offer and how chemistries like silicone have evolved over time. In 1963 a patent invention was filed for the technology of aqueous organopolysiloxanes, which included their use with organic resins.3 The organopolysiloxane components were recommended to be used in a coating composition to increase chalk resistance, smudge resistance, weather resistance, water repellency and have excellent air and water vapor permeability.3 Patented silicone resin emulsions have been successfully used in paints for over 50 years. Within those 50 years, innovative advancements in waterborne silicone technology continue to increase the technology’s strong advantages.
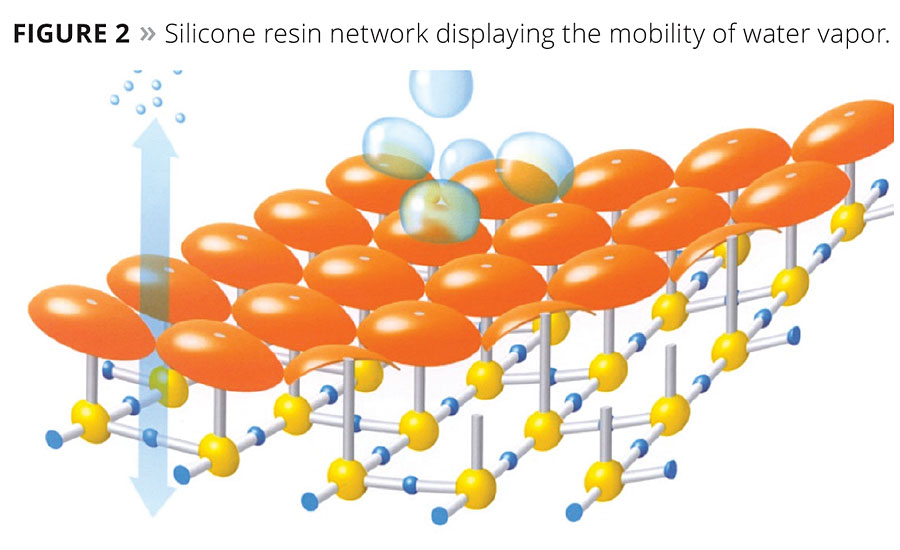
Silicone resins are built as methyl silicone resins containing silicone difunctional and trifunctional silicone units. As described above, the selection of using D and T silicone units determines the hardness and flexibility of the resin. Organofunctionality is then added to increase water repellency, alkaline stability and increase compatibility with organic components. Alkoxy groups are then responsible for reactivity, crosslinking, adhesion to substrates and coating cohesion. The contributing chemistries then form a silicone resin network system, displayed in Figure 2. The network is then formed into a hydrophobic shell that does not allow water into the film, but creates hydrophobic pores and capillaries that allow the coating film to have water vapor permeability. The silicone resin micelles increase the hydrophobicity of the film by protecting the surface from the degrading environment. Silicone’s ability to protect against UV light provides key advantages over other binders. The increased UV protection is built into the backbone of the structure with a silicon oxygen bond (Si-O-Si), which has a bond strength of approximately 110 kcal/mol.4 The bond strength for a C-C bond is approximately 85 kcal/mole and 87 kcal/mol for a C-O bond.4 This strength allows for little additions of silicone to be used and physical properties to be visible. Overall, the silicone resin network increases the hydrophobicity of the coating film to reduce dirt pick up, creating a moisture-resistant film and increasing the film’s long-term durability.
In today’s marketplace, silicone resin emulsions can participate as a sole binder or a resin modifier within a coating formulation. More commonly, silicone resin emulsions are found as resin modifiers due to their cost and beneficial performance at lower loading levels. The silicone resin disperses throughout the coating and envelops the fillers and pigments while the organic binder acts as glue between the filler particles and substrate.5 The silicone increases water repellency while reinforcing the film’s porosity.5 The mechanism of the silicone’s behavior in a paint system in comparison to an organic binder is displayed in the micrographs in Figure 3. At high magnification, small menisci between mineral particles indicate the presence of the silicone resin network. The silicone network uniformly covers the nanoscale TiO2 and other fillers with extremely thin films that strengthen the paint’s microstructure in the nanometer range while preserving its micro porosity. The additives give additional support by partially penetrating into the coating and align more at the surface of the film to increase water repellency and visual early water beading.
It is important to learn the effectiveness of the silicone modifiers in terms of performance time after coating application. Silicone emulsion binders require time to develop into the coating, while lower-molecular-weight silicone additives reduce water uptake almost immediately after the application. The essential task for the additives besides early water uptake is also to increase water contact angle. Surfactants or dispersants can decrease the initial silicone effect at the surface of the coating, masking the silicone performance. UV and water exposure of the film can increase the speed of removal for the surfactant release from the coating while increasing the silicone performance during that time.
Silicone additives used in coatings are commonly formulated with difunctional units that allow for flexibility and have reactive compounds that increase water repellency. The trifunctional modifications increase the hardness and binding power with more crosslinking. The molecular weight and active groups can determine the additives’ compatibility with the surface. The additive silicone materials have low viscosity with excellent stability themselves and in a coating. Their performance is dependent on their low surface tension and hydrophobic components that can vary water beading contact angles. Modifications to the organofunctionality produce key advantages, especially if amino-functional polysiloxanes are used. Advantages to formulating additives with amino-functional polysiloxane chemistries to be used in coatings using the polydimethylsiloxane (PDMS) chemistry are durability, water repellency, UV stability, decrease in surface defects, and long-lasting performance after years of exposure. Additives containing amino-functional polysiloxane chemistries have a higher affinity for the substrate, also improving adhesion. The tendency for this type of chemistry to wash out of the coating does not exist versus the standard PDMS technology. In addition, the amino-functional polysiloxanes are also recoatable due to their alkyl functionality, which improves the surface energy that allows this advantage. The organic functionality and unique emulsification system allow for easier formulation use in the coatings market, with the flexibility to be used in various types of organic binder systems, including acrylic, polyesters, alkyds, polyethers, hybrids, polyurethanes, etc. There are numerous silicone additive chemistries that are commercially available, but to reduce the testing size the selected few for this evaluation were as follows: silane/siloxane blend, silane emulsion and two different forms of reactive emulsions containing amino-functional polysiloxane.
Silicone Exterior Exposure Testing
There are those who are not convinced of silicone’s true advantages in the great outdoors and their reduction in dirt pick up. To prove that silicones work, three formulations were used with multiple silicone binders and silicone additives at 5% and 10% loading levels. Performance of all raw materials investigated was the same across all three formulations. Therefore, results from only one formulation are shown (Table 1). The formulation was built with basic coating raw materials with a PVC level of 35%. The formulation contained a control; styrene acrylic. The formulation was made in large scale, then silicone raw materials were post added to minimize variability. Dosage amounts of 5% and 10% were used, as seen in Table 1. Silicone’s ultimate performance is typically seen if the silicone is incorporated earlier into the formulation after the grind phase and before the acrylic letdown to envelop the fillers and pigments as described previously. Post additions are used for initial screening of the performance material. If benefits can be seen with post addition, then it will also work well when added earlier in the formulation. More in-depth screening can be performed after narrowing down of sample chemistry and choosing an optimized loading level.
The paints were then coated onto sections of 36-inch-long southern yellow pine siding boards and smooth aggregate concrete panels. Two coats were applied and left to dry for seven days before initial color and water absorption data were collected. Color and gloss readings were performed using a BYK spectro-guide. For the concrete panels, water absorption data was collected following a similar procedure to ASTM D5401 where the panels are placed face down onto a deionized water-soaked sponge.
Weights of the concrete panels were taken initially, then after soaking 1 h, 6 h and 24 h to determine the amount of water absorbed by the film. The data was then analyzed to provide feedback on the optimal performance of the coating. This was done initially and every six months. Two out of the three concrete and wood panels were placed outside in Michigan at a 45° angle, south-facing exposure. One panel was kept inside as a control to compare initial color and water absorption with no exposure.
Analysis of Silicone Performance
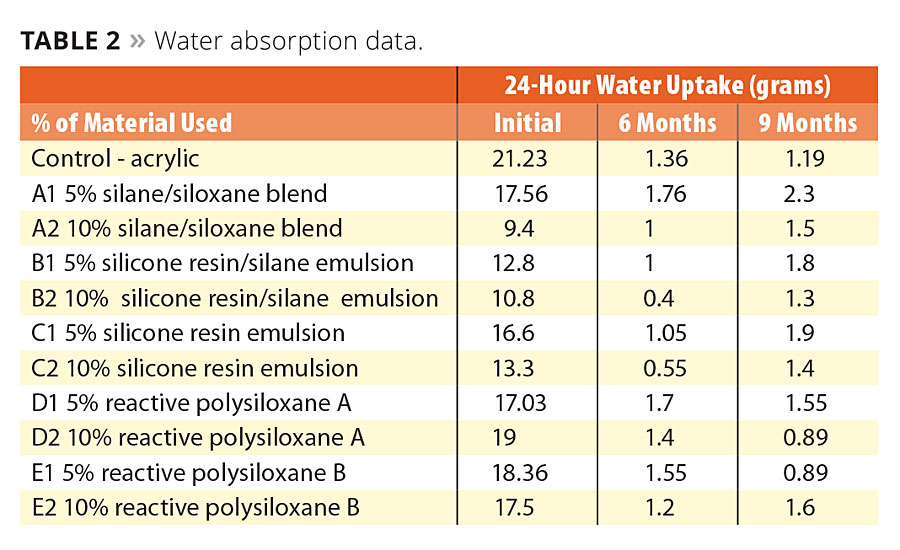
After collecting three sets of data over the course of nine months there was enough data to determine the silicone’s performance value. Table 2 shows the water absorption data after nine months exposure. The coated concrete panels absorbed less water after six months than they did initially, which is due to the films’ need to equilibrate by washing out water-soluble emulsifiers of the panels. The data verifies that this particular acrylic had a decline in water absorption with exposure time. Almost every technology evaluated in this study showed that the more silicone that was added to the formulation, the less water was absorbed overall. This does not mean this always holds true. For some silicone technologies there can be a critical level to where more does not equal better results. The information gained through evaluations and data collection increases our knowledge of how to modify the silicone chemistry for further optimization.
Photos captured the degree of dirt pick up after nine months of exposure. The difference in the degree of dirt pick up was based on the difference of chemistry and silicone chemistry. The acrylic as the control picked up a moderate amount of dirt. The silane/siloxane blends at both loading levels had the most significant dirt pick up with streak marks. Both reactive polysiloxanes at 5% loading levels had slightly moderate dirt pick up, while the 10% loading levels had moderate to heavy dirt. Results indicate that the loading levels are important, and verify that a softer silicone additive performs well at lower loading levels but reaches a threshold. The silicone resin/silane emulsion showed slight dirt pick up. The silicone resin emulsion had the least dirt pick up; showing very little color change over the nine-month period at both loading levels. The results physically demonstrate that the benefits of particular silicone chemistry added to an exterior formulation can be seen in as little as nine months. It is hard to see the magnitude of improvement from the photos, but the most observable panels are displayed in Figures 4, 5 and 6. In Figure 4 the concrete panels are displayed without the indoor controls at 10% silicone loading level in comparison to the acrylic control. In Figure 5 the concrete panels are displayed at 5 and 10% silicone loading levels with their controls (no exposure) to compare the initial color to the nine months discoloration of the panel. Figure 6 displays the southern yellow pine coated panel after nine months of exposure. The wood and the concrete both display similar results.
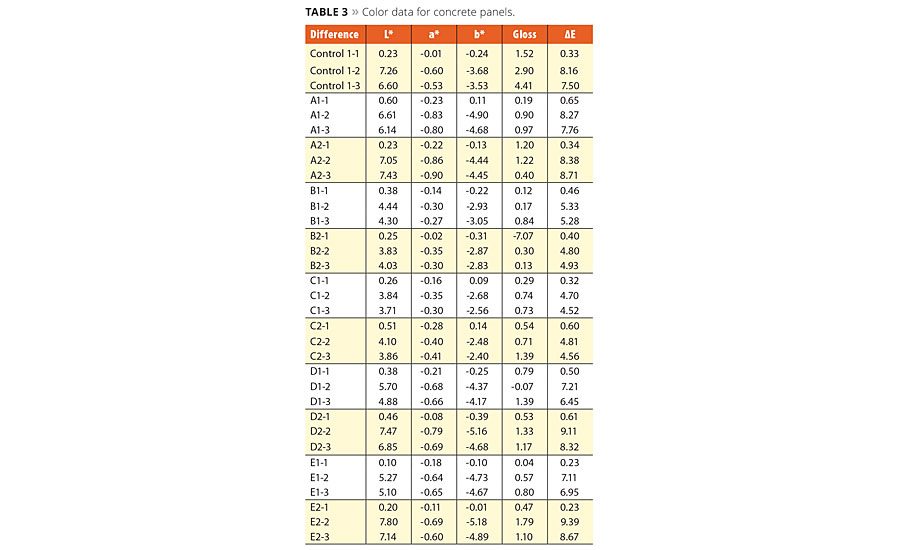
To verify the color change, the color values including the delta E have been included in Table 3. The first panel is the control that stayed inside with no UV exposure. Panels 2 and 3 of each evaluated technology are the exterior panels. As you will see, the higher the delta E values the more color change. In ranking them from least dirt pick up to most dirt pick up it is easy to align with the photos and color data; silicone resin emulsion, silicone resin/silane emulsion, low loading levels of reactive polysiloxanes, followed by the high loading levels of reactive polysiloxane, silane/siloxane blend and the acrylic. It verifies that the instrumental readings match the visual appearance.
Conclusion
Certain silicone technology, like silicone resin emulsions, can provide a significant decrease in dirt pick up from exposure due to the hydrophobic properties. The outdoor exposure data collection for the formulations and panels shown will continue for years to verify the true advantages of the silicone performance. As clean surfaces are a significant consumer demand, this study provides efficient results of how silicone can meet consumer’s needs. This initial work provides answers to begin more exploratory work. More work is necessary to narrow down further silicone chemistries and have confidence to deliver a clear silicone technology response to which silicone building block technology provides the best dirt pick up solution.
Acknowledgements
The author gratefully acknowledges support from James Greene, Andrew Pearson and Global SC colleagues of Wacker Chemical Corporation. Note: All figures and tables are property of Wacker Chemical Corporation
References
1 Hunter, B. Big-Ticket Remodeling Activity Grows 3.3% in 3Q, Remodeling 2014, http://www.remodeling.hw.net, -January 8, 2015.
2 Heldman, D.K.; Larzelere, K. K.; Greene, J.D. Reaching Strict VOC Requirements with Outstanding Durability for Industrial Maintenance and Marine Coatings, Paint & Coatings Industry 2008, 34-43.
3 Nitzsche, S.; Pirson, E.;Roth, M. Coating Agents, US Patent, US 3,294,709 December 27, 1966.
4 Andrews, A. Benefits to Using Silicone Hybrids for Improvements to Wood Coatings, Waterborne Symposium, 2013.
5 Andrews, A. Silicones are the Innovative Solution for Architectural Coatings, Waterborne Symposium, 2015.
This paper was presented at the CoatingsTech Conference in Louisville, KY, March 9-11, 2015.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!