Fumed silica, also known as pyrogenic silica, is an important additive used to modify performance characteristics in a variety of coating formulations. Due to its high purity, small particle size and high surface area, fumed silica serves as a thickener, viscosity modifier, pigment extender and free flow agent. However, unsustainable manufacturing processes are used to produce fumed silica, creating additional cost, liability and environmental impact.
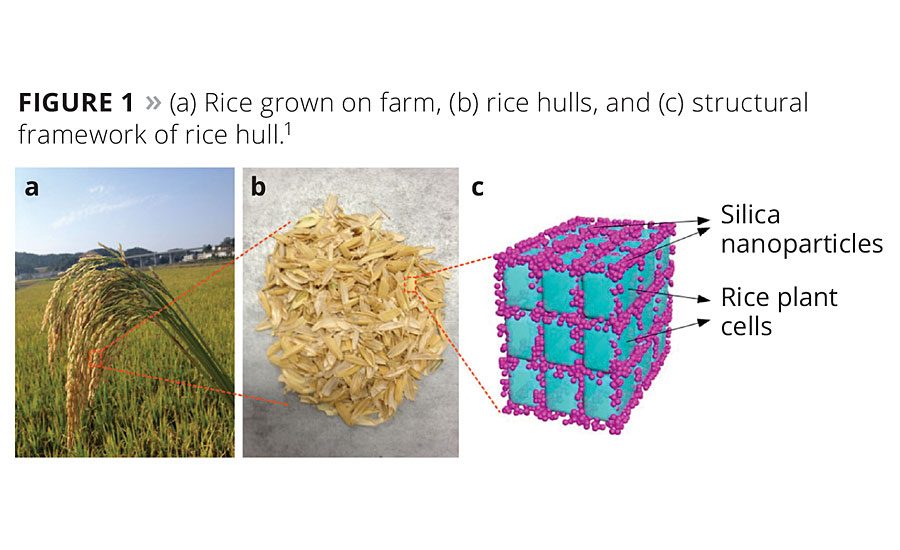
According to an online survey at www.pcimag.com, many paint manufacturers consider sustainability to be indispensable or very important when it comes to the manufacturing choices that they make. SioTeX Corporation has developed a sustainable alternative to provide manufacturers with a viable alternative to traditional fumed silica. Harvested from rice hulls, biogenic silica (-Eco-Sil™) exhibits the high purity, small particle size and high surface area that are necessary to achieve desired performance. Rice hulls possess an inherent structural framework of silica nanoparticles, as shown in Figure 1.
Statement of the Problem
Fumed silica producers rely on a costly, energy-intensive and hazardous manufacturing process. The air pollution resulting from the energy utilized to produce fumed silica is a combination of carbon emissions from the production of raw materials, energy generation and overseas transportation, as well as corrosive and toxic hydrogen chloride gas from the process.
Millions of tons of rice hulls are produced every year, but most of the agricultural biowaste is burned or landfilled. The combustion of rice hulls releases large quantities of carbon dioxide. When they decompose in a landfill, the rice hulls produce methane, a greenhouse gas that is more harmful than carbon dioxide.
Additionally, coatings products are competitively priced, which leads manufacturers to seek cost-saving alternatives. Biogenic silica solves both of these problems due to its inexpensive and abundant raw material and efficient manufacturing processes.
Material Characteristics
Biogenic silica is obtained by the complete combustion of rice hulls that have undergone a proprietary chemical treatment. It has been characterized for purity, crystallinity, surface area and microstructure. Extensive testing of the biogenic silica shows that it has desired characteristics. Additionally, its higher bulk density results in improved handling, packaging and storage.
Chemical Composition
The traditional combustion of rice hulls yields rice hull ash, a grayish-black crystalline product that contains 5% to 10% of unburned carbon material and other mineral oxides, such as potassium oxide, calcium oxide and sodium oxide.2 In this case, the usefulness of rice hull ash is limited to applications where color, crystallinity and high purity are not important.
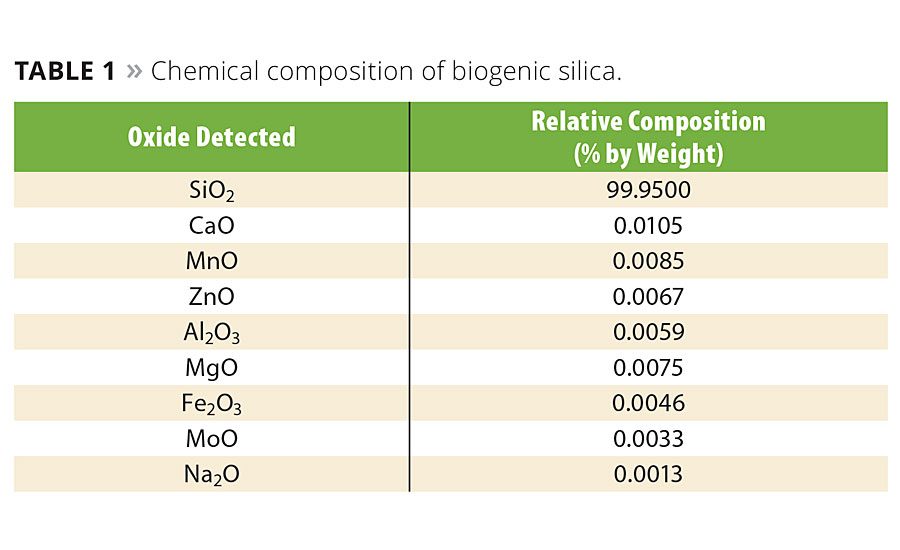
The complete combustion of carbon and reduction in the content of alkali, alkaline earth and transition metals results in a high-purity silica with expanded application potential. Chemical analysis was conducted to determine the purity of the silica using inductively coupled plasma optical emission spectrometry (ICP-OES). The analytical instrument used was a Varian Liberty Series II Spectro-meter. The sample was dissolved in a 1:1:1 mixture of 50% HF, HNO3 and HCl at 80 °C. The concentrations by mass (mg/kg) of analytes were converted to weight percent. Table 1 shows the results of the ICP-OES test. The amorphous silica content was 99.95% with only trace quantities of other mineral oxides.
Crystallinity
The presence of crystalline silica is not allowed in products because it is a known carcinogen.3 The chemical pretreatment carried out prior to combustion prevents the formation of crystalline silica resulting from phase changes.4
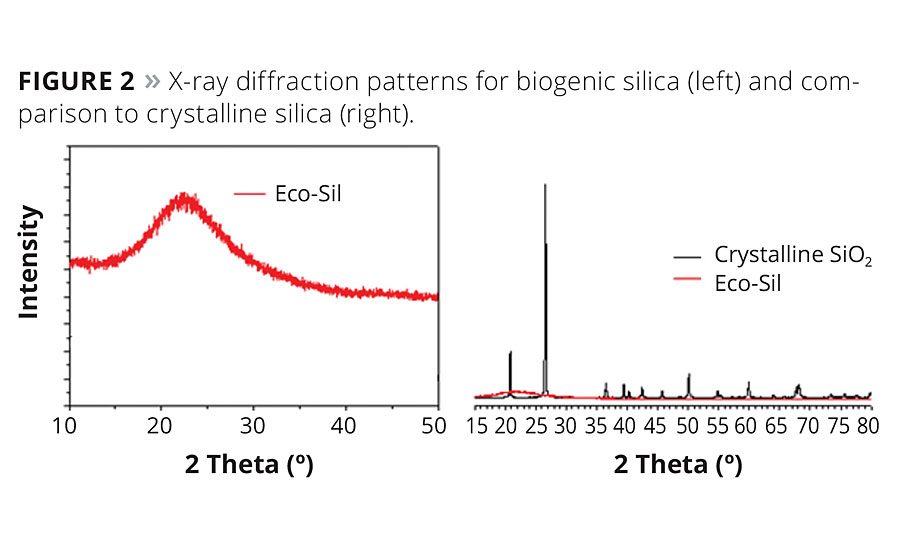
X-ray diffraction (XRD) on the biogenic silica in its powder form was performed with a Cu-Kαsource. No evidence of crystallinity was detected. The 2-theta region between 10° to 50° at long collection times indicates no crystalline peaks. Crystallinity above 0.01% by weight would be visible as sharp peaks on the diffraction pattern. Figure 2 illustrates the amorphous diffraction pattern for biogenic silica and compares it to that of crystalline silica. Note the sharp peaks at 21.9° and 26.4° 2-theta, indicating quartz and cristobalite crystal forms.
Surface Area
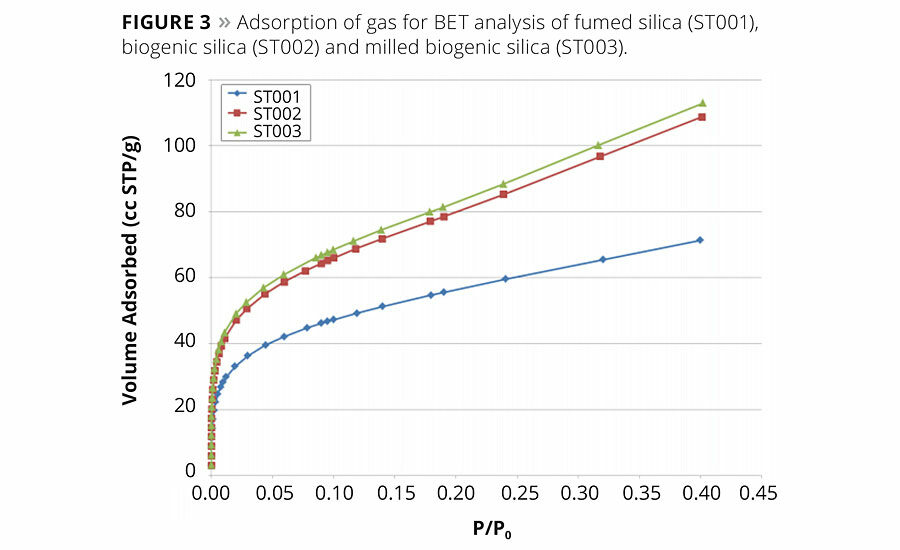
The porosity and surface area of the material play a critical role regarding its reactivity and use as a viscosity modifier. Materials with high surface area outperform those with lower surface area. The Brunauer, Emmett and Teller (BET) theory for adsorption of gases on the surfaces of solids is the industry standard for determination of specific surface area. The surface area is typically reported in square meters per gram of the sample. Figure 3 shows the plot of N2 adsorbed as a function of pressure. The higher volume and initial steep slope indicate that the biogenic silica adsorbs gases more readily than fumed silica. The surface area of the biogenic silica is 294 m2/g compared to a range of 100 m2/g to 400 m2/g for commercially available fumed silica.
Microstructure
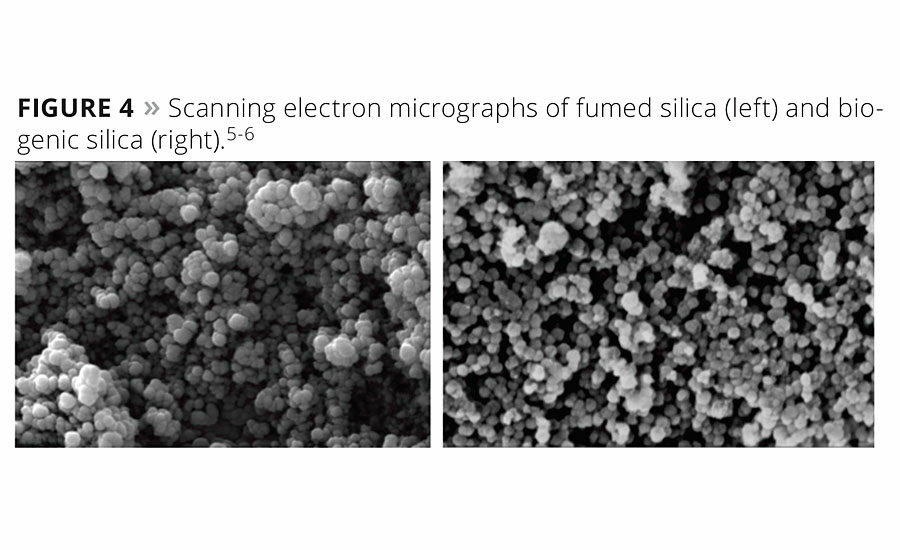
Scanning electron microscopy was conducted to qualitatively image the material and compare it to commercial synthetic amorphous fumed silica. Both materials exhibit a similar morphology consisting of branched silica nanoparticles (Figure 4).
Performance
Viscosity
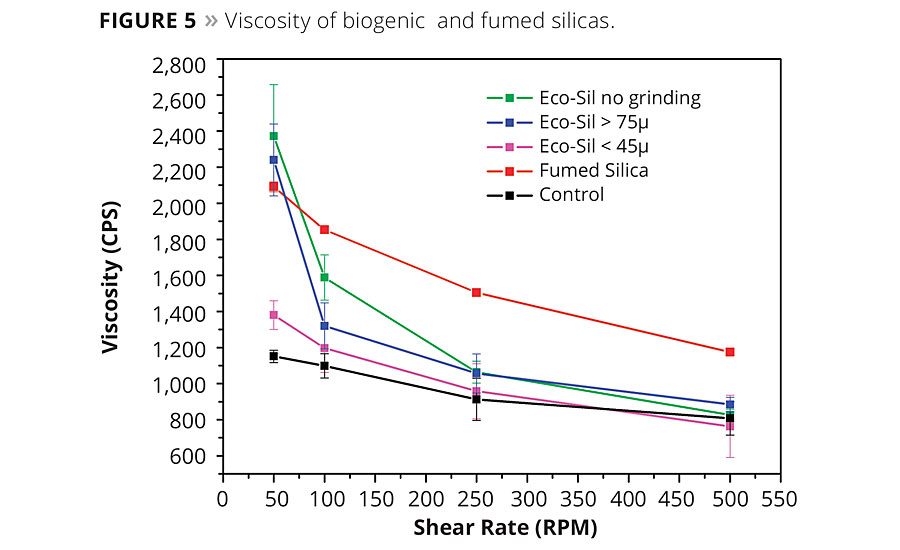
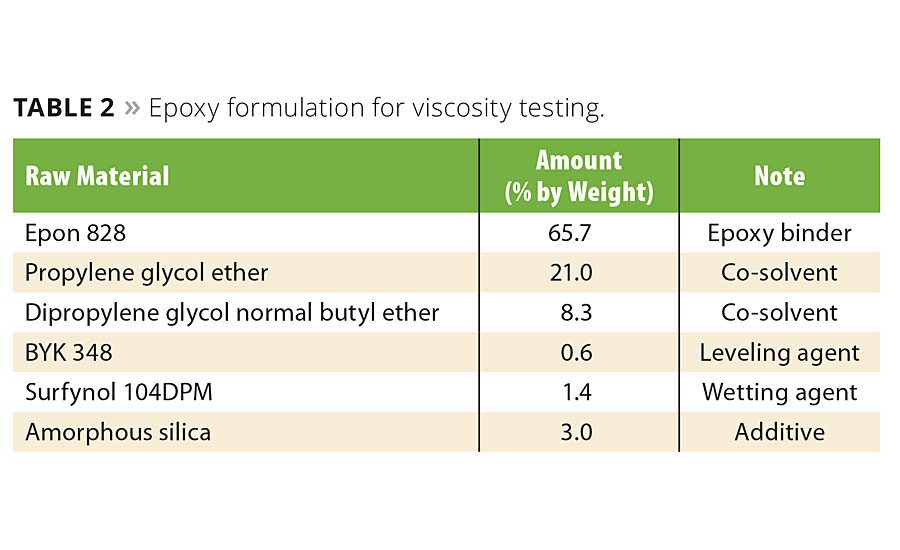
It is well known that one of the most important uses of specialty silica is thixotropic viscosity modification.7-9 The shear-thinning performance of the biogenic silica was evaluated in the epoxy formulation found in Table 2. As a model system, a comparison was made between biogenic silica and fumed silica. The formulations were mixed for 10 min at 2000 rpm with a Dispermat D-51580 and degased for 10 min prior to viscosity measurement. Viscosity was measured with a Brookfield Cap 2000+ Viscometer at 20 °C. In Figure 5, both materials displayed thixotropic behavior in the epoxy coating. The thixotropic index for the biogenic silica and the fumed silica in the model system were about 2.8 and 1.8, respectively. Excessive shear before use can hinder its viscosity performance. It is speculated that excessive shear may cause damage to the microstructure of biogenic silica.
Thickening
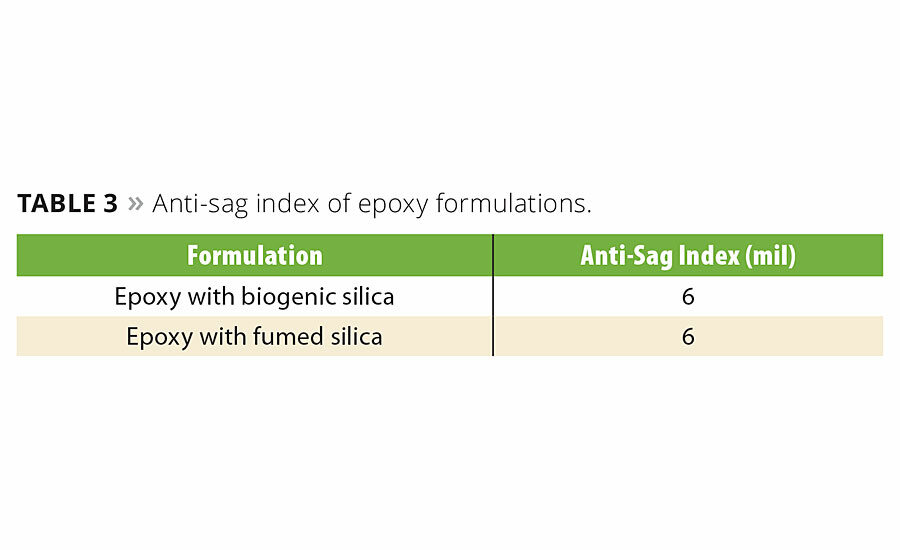
The sag resistance of biogenic silica and fumed silica in the epoxy coating formulation was measured, and results are shown in Table 3. The sag resistance of the biogenic silica and fumed silica formulas was similar.
Conclusions
The characteristics and performance of Eco-Sil harvested from rice hulls indicate that it is a cost-effective and environmentally acceptable replacement for fumed silica. Alternatives to fumed silica based on green technology will allow the coatings industry to incorporate a sustainable and competitively priced silica additive.
References
1 Liu, N.; Huo, K.; McDowell, M.; Zhao, J.; Cui, Y. Rice husk as a sustainable source of nanostructured silicon for high performance Li-ion anodes. Scientific Report, 3, 2013.
2 Kumar, A.; Mohanta, K.; Kumar, D.; Parkash, O. Properties and industrial applications of rice husk: A review. International Journal of Emerging Technology and Advanced Engineering2012, 2 (10), 86-90.
3 Steenland, K.; Sanderson, W. Lung cancer among industrial sand workers exposed to crystalline silica. American Journal of Epidemiology2001, 153 (7), 695-703.
4 Armesto, L.; Bahillo, A.; Veijonen, K.; Cabanillas, A.; Otero, J. Combustion behaviour of rice husk in a bubbling fluidised bed. Biomass and Bioenergy2002, 23, 171-179.
5 Binks, B. P.; Fletcher, P. D.; Holt, B. L.; Beaussoubre, P.; Wong, K. Phase inversion of particle-stabilised perfume oil-water emulsions: Experiment and theory. Physical Chemistry Chemical Physics2010, 12 (38), 11954-11966.
6 Chen, H.; Wang, W.; Martin, J. C.; Oliphant, A. J.; Doerr, P. A.; Xu, J. F.; Sun, L. Extraction of lignocellulose and synthesis of porous silica nanoparticles from rice husks: A comprehensive utilization of rice husk biomass. ACS Sustainable Chemistry & Engineering2013, 1 (2), 254-259.
7 Yziquel, F.; Carreau, P. J.; Moan, M.; Tanguy, P. A. Rheological modeling of concentrated colloidal suspensions. Journal of Non-Newtonian Mechanics1999, 86 (1-2), 133-155.
8 Gunko, V. M.; Zarko, V. I.; Leboda, R.; Chibowski, E. Aqueous suspension of fumed oxides: Particle size distribution and zeta potential. Advances in Colloid Interface Science2001, 91, 1-112.
9 Litchfield, D. W.; Baird, D. G. The rheology of high aspect ratio nano-particle filled liquids. Rheology Reviews2006, 1-60.
For more information, contact Lisa Taylor at 512/762.9872 or email lisa.taylor@sio-tex.com.
By Haoran Chen, Ph.D.; Chief Technical Officer; Marcus Goss, Chief Operations Officer; and Ash Kotwal, Vice President of Manufacturing | SioTeX® Corp., San Marcos, TX