A New Development in Penniman Red Production
A Solution for Nitrous Oxide Emissions








With annual requirements of around one million metric tons, synthetic iron oxide pigments are the largest group of colored inorganic pigments. Their high weathering resistance, resistance to bleaching sunlight, and their warm, natural shades make iron oxide pigments very important raw materials for the coatings industry. Iron oxide red, also known as hematite, forms the most important subgroup. Applications for iron oxide red pigments include the coloring of building materials, plastics, and coatings.
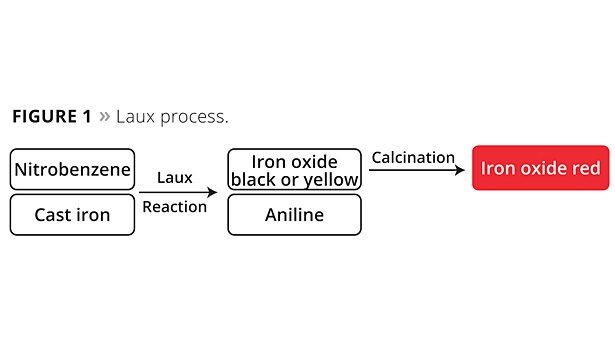
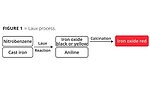
Four processes in particular are used worldwide for the industrial production of iron oxide red pigments. The Laux process oxidizes iron with nitrobenzene. Yellow or black iron oxide pigments are formed in this process. The latter in turn can be converted to particularly high-quality red pigments through a calcination stage. Aniline is formed as a by-product of the iron oxide pigments and must be completely removed from the process wastewater. In addition to phase separations, this also requires effective biological cleaning to remove residual quantities efficiently and reliably. In the downstream calcination process necessary for producing iron oxide red pigments, small quantities of sulfur oxides are released and these need to be removed from the waste gas stream through a cleaning process. Starting with black iron oxide, also known as magnetite, an oxidation process takes place at temperatures of ~800 °C. Due to the exothermic oxidation reaction from magnetite to hematite, only very little energy in the form of heat is introduced in this step. Even when viewed overall, the Laux process (Figure 1) requires a particularly small amount of energy compared to all other iron oxide red processes due to the powerful oxidation effect of nitrobenzene and the release of reaction heat that can be used in the process.
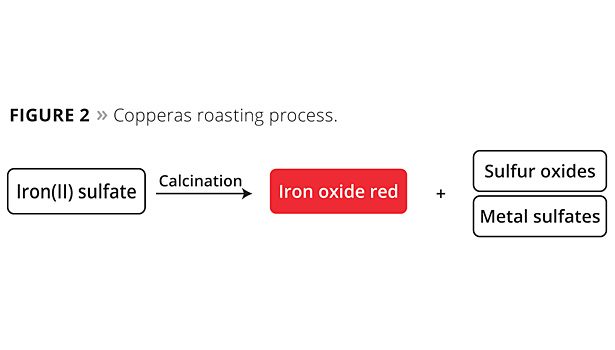
The copperas process involves direct roasting of iron sulfate at high temperatures with stoichiometric quantities of sulfur oxides being separated off (Figure 2). These oxides are extremely problematic by-products, as their corrosiveness and toxicity require intensive – and suitably complex – waste gas cleaning. The iron oxide pigments formed during the roasting process still contain various soluble metal sulfates that need to be removed subsequently in an aqueous washing process before the pigment can be dried again. The wastewater contains iron(II) sulfate that has not been converted, together with other soluble heavy metal sulfates, and requires very complex cleaning or needs to be disposed of.
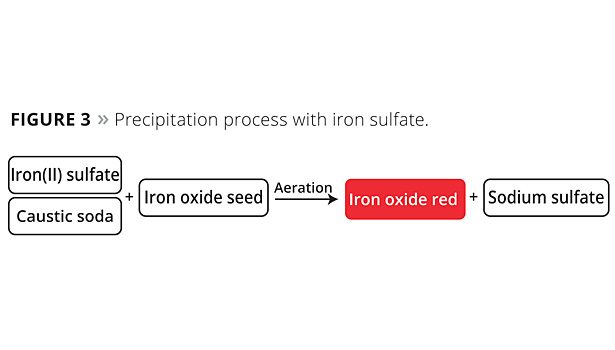
The precipitation process begins from iron(II) sulfate (Figure 3) or chloride, with aqueous precipitation being performed under oxidative conditions using a lye such as a sodium hydroxide solution. Suitable iron oxide seeds are also added to ensure uniform pigment development. Given the high temperature required for the precipitation reaction, this process is particularly energy-intensive compared to the other processes and also requires immense volumes of water. The stoichiometric reaction produces particularly large quantities of salt (e.g., sodium sulfate) in the wastewater. An energy-intensive water evaporation stage is required to remove this salt from the water.
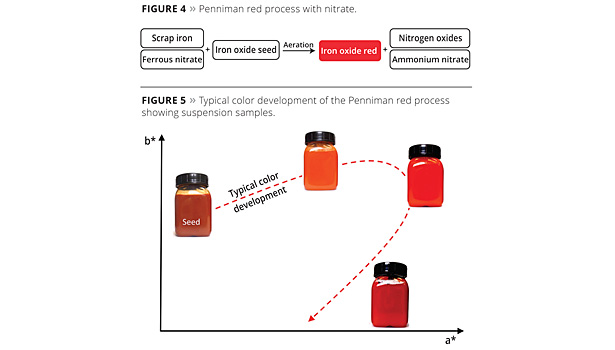
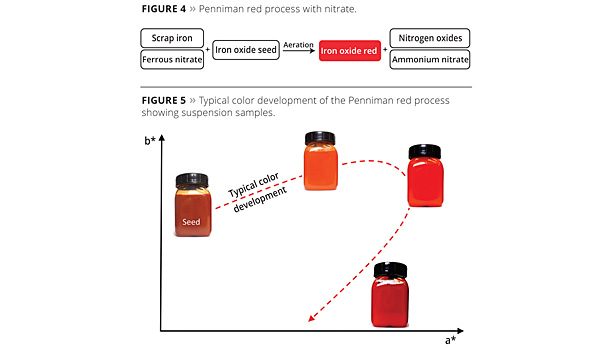
Using scrap iron, hematite seeds, ferrous nitrate and aeration, the Penniman red process is used to produce particularly yellowish red pigments. A reaction diagram is shown in Figure 4. One problem with this process, however, is the formation of toxic and environmentally harmful nitrogen oxides and wastewater containing dissolved ammonium nitrate.
Challenges
All four processes for producing iron oxide red pigments pose specific challenges when it comes to achieving sustainable, resource-friendly production. Various aspects need to be factored in, such as the specific energy and water requirements, and the treatment of waste gases and wastewater. What’s more, all the processes described have their strengths and weaknesses regarding the target color space. While the Laux process, for example, is highly suitable for producing high-quality medium and dark red pigments (Bayferrox® 120 or Bayferrox® 180), it exhibits weaknesses when used for manufacturing particularly yellowish red pigments. The Penniman red process in particular has exceptional strengths in this color space, however.
The diverging target color spaces are due, among other things, to differences in the particle morphology and particle size distribution. The exact color parameters cannot, however, be predicted using a direct correlation with the particle morphology or particle size distribution. Particularly light, yellowish red pigments tend to exhibit a smaller particle size and an especially narrow particle size distribution, while darker red pigments are significantly larger. With the Penniman red process, suitable iron oxide seeds are used that grow slowly and uniformly into the pigment. The fact that the pigment particles grow uniformly means the process can be stopped at any time to achieve any desired color shade on the color development profile. The Penniman red process is therefore particularly suitable for producing light red pigments with high chromaticity.
During the color development process, the color can be seen to change during the course of the reaction. While the fine hematite seeds are brown and exhibit transparent color characteristics, they take on an increasingly red coloring during the pigment growth reaction, and their degree of opacity increases. After the a* value (red, CIELAB) has reached a maximum that is dependent on the process parameters, the yellow (b*) and red (a*) components then decrease again and the pigment becomes ever darker (Figure 5). This process is therefore very flexible when it comes to attaining various color loci, from yellowish to dark red grades. Given the long reaction time before the dark grades (e.g., Bayferrox 160 or Bayferrox 180) are reached, however, calcination processes such as the Laux process are better suited for this target color range. With the yellowish red pigments, however, the Penniman process is particularly suitable since the color development can be managed very effectively.
Just like the other significant iron oxide red processes, the Penniman red process faces specific challenges when it comes to sustainable and environmentally friendly production. With annual production figures of around 300,000 metric tons, this technology is one of the most important processes for producing iron oxide red pigments. Global Penniman red production is focused entirely on production facilities in China.
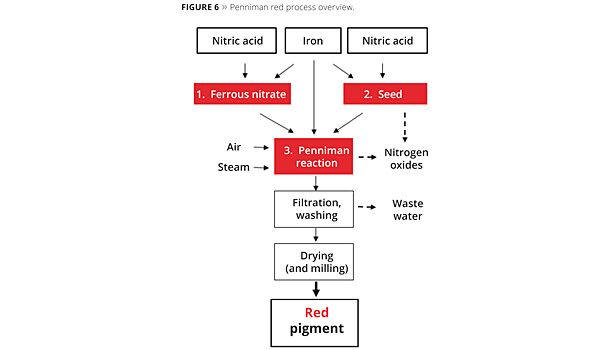
The Penniman red process consists of three elements. Starting with nitric acid and scrap iron, a hematite seed is produced at temperatures of over 90 °C. Fairly large quantities of nitrogen oxides are produced during conversion in this stage of the process. Ferrous nitrate that is required as an electrolyte for the subsequent Penniman reaction is produced in a further process step. As with hematite seed production, scrap iron is converted with nitric acid. For highly selective reactions, a reaction temperature of 60 °C should not be exceeded.
The actual Penniman development process is then performed. The hematite seed, together with the scrap iron and ferrous nitrate, is developed into the pigment at temperatures of 70-95 °C under aeration. As with hematite seed production, nitrogen oxides are released in this process step. The wastewater filtered off after pigment development contains dissolved nitrogen compounds such as ammonium and nitrate. To prevent thixotropic behavior of the pigments during filtration and thus to minimize water consumption during washing, sulfate is often added as a flocculant in the reaction solution. The filtered and washed iron oxide red pigment is then dried and, where applicable, micronized through milling (Figure 6).
The formation of gaseous nitrogen oxides (NOx), soluble nitrate and ammonium compounds in the wastewater and relatively high energy and water requirements make the Penniman red process a particularly complex and challenging technology from an ecological perspective.
A New Penniman Red Process
LANXESS has addressed these challenges and developed an innovative and sustainable Penniman red process. The development work surprisingly revealed that, in addition to the known nitrogen oxide emissions, significant quantities of laughing gas (nitrous oxide, N2O) are formed. As far as we understand, this fact was previously not known about the Penniman red process. Nitrous oxide is an extremely critical greenhouse gas – 300 times more potent in its effects than carbon dioxide, which already enjoys notoriety. It is also a compound that is depleting the ozone layer in the stratosphere. Unlike toxic nitrogen monoxide and nitrogen dioxide that are also formed, it cannot be removed through normal gas scrubbing. This means that, when using the typical Penniman red production process, it is currently released into the atmosphere untreated.
Given the serious effects this has on the climate and the extent of global Penniman red production, this represents a sizable contribution to global greenhouse gas emissions. To produce a single metric ton of red pigment, nitrous oxide, with a greenhouse effect averaging 15-20 metric tons of CO2 equivalents, is released.
In addition to the waste gas composition, the wastewater from the Penniman red reaction also constitutes an environmental load as it contains dissolved nitrogen compounds such as ammonium and nitrate. These can lead to an oversupply of nutrients when discharged into rivers and other bodies of water, which in turn can result in greater algae growth. This process, which is also known as eutrophication, ultimately also results in a shortage of oxygen in the water due to the high oxygen consumption occurring during the decomposition of vegetable matter (Figure 7). This advanced state of eutrophication leads in turn to the death of the fish population. Since the water is heavily contaminated by this process, this can have dramatic consequences for the drinking water supply. For instance, Lake Taihu in the Yangtze Delta region of China has been affected by eutrophication on multiple occasions as a result of industrial wastewater being discharged into the water.1 As China’s third largest reservoir of fresh water, this has immense consequences for fishing and the supply of drinking water in the region. At the same time, many industrial plants had to cease production temporarily. Iron oxide pigment manufacturers were among those affected.
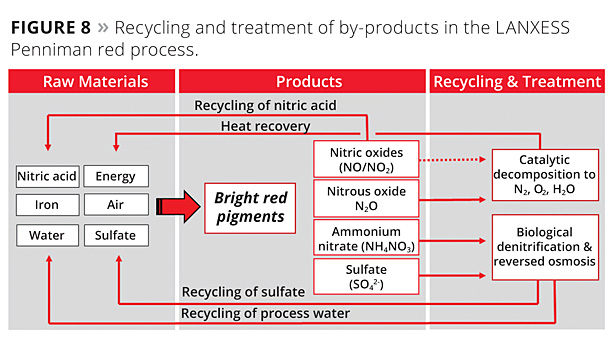
When developing a sustainable Penniman process that was in line with the principles of Green Chemistry,2 the avoidance of by-products was at the top of the agenda. Process optimization succeeded in cutting nitrogen oxide emissions during Penniman pigment development by over 90% while also reducing energy consumption by 80%. More effective use of ferrous nitrate significantly reduced the demand for this raw material, which in turn minimized the quantity of dissolved nitrogen compounds in the wastewater. Most of the residual nitrogen oxides that could not be prevented through process optimization were removed in an innovative nitric acid recovery plant, with the recovered nitric acid serving as a raw material for the process. Since nitrous oxide cannot be converted to nitric acid in this way, the waste gas stream of the nitric acid recovery plant contains even greater quantities of this compound. This nitrous oxide and traces of other nitrogen oxides that have not been washed out are degraded to naturally occurring nitrogen gas, oxygen and water by means of a catalyst. Since conversion of the gases at the catalyst is exothermic, the resulting reaction heat can be employed in the process through the use of heat exchangers (Figure 8).
The multistage wastewater treatment process uses sedimentation, biological denitrification, ultrafiltration and reverse osmosis. Over 80% of the wastewater is cleaned and can be reused directly in the process. The remaining 20% of the wastewater is practically free of dissolved nitrogen compounds and contains only dissolved sulfate. This can be fed back in full to the process. This process can thus be completed without the formation of any wastewater containing salt.
Conclusion
The innovative Penniman red process developed by LANXESS sets new standards in the iron oxide industry due to its very low NOx and nitrous oxide emissions made possible by comprehensive waste gas and wastewater cleaning. The company’s Inorganic Pigments business unit will employ this Penniman red technology, which is sustainable, particularly energy efficient and water conserving, in its new production facility in Ningbo, China. Coatings manufacturers will now be able to incorporate iron oxide red pigments into their formulations that are produced in a sustainable manner.
References
1 Qin, B.; Liu, Z.; Havens, K. Hydrobiologia 2007, 581, VIII, 328.
2 Anastas, P.T.; Warner, J.C. Green Chemistry: Theory and Practice, Oxford University Press: New York, p. 30, 1998.
By Dr. Waldemar Czaplik, Research Manager, Inorganic Pigments, LANXESS Deutschland GmbH, Krefeld-Uerdingen, Germany
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!